Cellular and Genetic Causes of Idiopathic Hyperaldosteronism
- PMID: 30354720
- PMCID: PMC6207209
- DOI: 10.1161/HYPERTENSIONAHA.118.11086
Cellular and Genetic Causes of Idiopathic Hyperaldosteronism
Abstract
Primary aldosteronism affects ≈5% to 10% of hypertensive patients and has unilateral and bilateral forms. Most unilateral primary aldosteronism is caused by computed tomography-detectable aldosterone-producing adenomas, which express CYP11B2 (aldosterone synthase) and frequently harbor somatic mutations in aldosterone-regulating genes. The cause of the most common bilateral form of primary aldosteronism, idiopathic hyperaldosteronism (IHA), is believed to be diffuse hyperplasia of aldosterone-producing cells within the adrenal cortex. Herein, a multi-institution cohort of 15 IHA adrenals was examined with CYP11B2 immunohistochemistry and next-generation sequencing. CYP11B2 immunoreactivity in adrenal glomerulosa harboring non-nodular hyperplasia was only observed in 4/15 IHA adrenals suggesting that hyperplasia of CYP11B2-expressing cells may not be the major cause of IHA. However, the adrenal cortex of all IHA adrenals harbored at least 1 CYP11B2-positive aldosterone-producing cell cluster (APCC) or micro-aldosterone-producing adenomas. The number of APCCs per case (and individual APCC area) in IHA adrenals was significantly larger than in normotensive controls. Next-generation sequencing of DNA from 99 IHA APCCs demonstrated somatic mutations in genes encoding the L-type calcium voltage-gated channel subunit α 1-D ( CACNA1D, n=57; 58%) and potassium voltage-gated channel subfamily J-5 ( KCNJ5, n=1; 1%). These data suggest that IHA may result from not only hyperplasia but also the accumulation or enlargement of computed tomography-undetectable APCC harboring somatic aldosterone-driver gene mutations. The high prevalence of mutations in the CACNA1D L-type calcium channel provides a potential actionable therapeutic target that could complement mineralocorticoid blockade and inhibit aldosterone overproduction in some IHA patients.
Keywords: adrenal cortex; aldosterone; calcium channels; hyperaldosteronism; hypertension.
Conflict of interest statement
Conflicts of Interest/Disclosures Statement
S. A. T. is supported as the A. Alfred Taubman Emerging Scholar by the A. Alfred Taubman Medical Research Institute. S. A. T. has received travel support from Thermo Fisher Scientific and had a separate sponsored research agreement with Thermo Fisher Scientific. None of the study described herein was supported by Thermo Fisher Scientific and they had no role in the data collection, interpretation, or analysis, and did not participate in the study design or the decision to submit for publication. The remaining authors have declared that no conflict of interest exists.
Figures


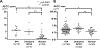
Comment in
-
Idiopathic Hyperaldosteronism.Hypertension. 2018 Oct;72(4):839-840. doi: 10.1161/HYPERTENSIONAHA.118.11174. Hypertension. 2018. PMID: 30354730 No abstract available.
References
-
- Merai R, Siegel C, Rakotz M, Basch P, Wright J, Wong B, Dhsc, Thorpe P. Cdc grand rounds: A public health approach to detect and control hypertension. MMWR Morb. Mortal. Wkly. Rep 2016;65:1261–1264 - PubMed
-
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB, American Heart Association Statistics C, Stroke Statistics S. Heart disease and stroke statistics−−2015 update: A report from the american heart association. Circulation. 2015;131:e29–322 - PubMed
-
- Kochanek KD, Xu J, Murphy SL, Minino AM, Kung HC. Deaths: Final data for 2009. Natl. Vital Stat. Rep 2011;60:1–116 - PubMed
-
- Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, Ganzaroli C, Giacchetti G, Letizia C, Maccario M, Mallamaci F, Mannelli M, Mattarello MJ, Moretti A, Palumbo G, Parenti G, Porteri E, Semplicini A, Rizzoni D, Rossi E, Boscaro M, Pessina AC, Mantero F, Investigators PS. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J. Am. Coll. Cardiol 2006;48:2293–2300 - PubMed
-
- Mulatero P, Stowasser M, Loh KC, Fardella CE, Gordon RD, Mosso L, Gomez-Sanchez CE, Veglio F, Young WF Jr. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J. Clin. Endocrinol. Metab. 2004;89:1045–1050 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

