Involvement of caveolin-1 in neurovascular unit remodeling after stroke: Effects on neovascularization and astrogliosis
- PMID: 30354902
- PMCID: PMC6928561
- DOI: 10.1177/0271678X18806893
Involvement of caveolin-1 in neurovascular unit remodeling after stroke: Effects on neovascularization and astrogliosis
Abstract
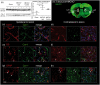
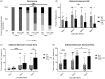
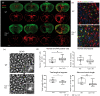
Complex cellular and molecular events occur in the neurovascular unit after stroke, such as blood-brain barrier (BBB) dysfunction and inflammation that contribute to neuronal death, neurological deterioration and mortality. Caveolin-1 (Cav-1) has distinct physiological functions such as caveolae formation associated with endocytosis and transcytosis as well as in signaling pathways. Cav-1 has been proposed to be involved in BBB dysfunction after brain injury; however, its precise role is poorly understood. The goal of this study was to characterize the expression and effect of Cav-1 deletion on outcome in the first week in a transient Middle Cerebral Artery Occlusion stroke model. We found increased Cav-1 expression in new blood vessels in the lesion and in reactive astrocytes in the peri-lesion areas. In Cav-1 KO mice, the lesion volume was larger and the behavioral outcome worse than in WT mice. Cav-1 KO mice exhibited reduced neovascularization and modified astrogliosis, without formation of a proper glial scar around the lesion at three days post injury, coinciding with aggravated outcomes. Altogether, these results point towards a potential protective role of endogenous Cav-1 in the first days after ischemia by promoting neovascularization, astrogliosis and scar formation.
Keywords: Caveolin-1; astrogliosis; ischemic stroke; neovascularization; neuroprotection.
Figures



References
-
- Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics – 2016 update: a report from the American Heart Association. Circulation 2016; 133: e38–360. - PubMed
-
- Meadows KL. Experimental models of focal and multifocal cerebral ischemia: a review. Rev Neurosci 2018; 29: 661–674. - PubMed
-
- Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. - PubMed
-
- Neuhaus AA, Couch Y, Hadley G, et al. Neuroprotection in stroke: the importance of collaboration and reproducibility. Brain 2017; 140: 2079–2092. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

