Thick Ascending Limb Sodium Transport in the Pathogenesis of Hypertension
- PMID: 30354966
- PMCID: PMC6335098
- DOI: 10.1152/physrev.00055.2017
Thick Ascending Limb Sodium Transport in the Pathogenesis of Hypertension
Abstract
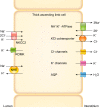
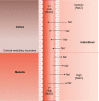
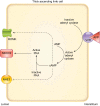
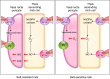
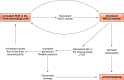
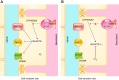
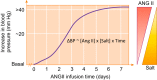
The thick ascending limb plays a key role in maintaining water and electrolyte balance. The importance of this segment in regulating blood pressure is evidenced by the effect of loop diuretics or local genetic defects on this parameter. Hormones and factors produced by thick ascending limbs have both autocrine and paracrine effects, which can extend prohypertensive signaling to other structures of the nephron. In this review, we discuss the role of the thick ascending limb in the development of hypertension, not as a sole participant, but one that works within the rich biological context of the renal medulla. We first provide an overview of the basic physiology of the segment and the anatomical considerations necessary to understand its relationship with other renal structures. We explore the physiopathological changes in thick ascending limbs occurring in both genetic and induced animal models of hypertension. We then discuss the racial differences and genetic defects that affect blood pressure in humans through changes in thick ascending limb transport rates. Throughout the text, we scrutinize methodologies and discuss the limitations of research techniques that, when overlooked, can lead investigators to make erroneous conclusions. Thus, in addition to advancing an understanding of the basic mechanisms of physiology, the ultimate goal of this work is to understand our research tools, to make better use of them, and to contextualize research data. Future advances in renal hypertension research will require not only collection of new experimental data, but also integration of our current knowledge.
Figures

Similar articles
-
Role of endothelin in thick ascending limb sodium chloride transport.Contrib Nephrol. 2011;172:76-83. doi: 10.1159/000328686. Epub 2011 Aug 30. Contrib Nephrol. 2011. PMID: 21893990 Review.
-
Sodium reabsorption in the thick ascending limb in relation to blood pressure: a clinical perspective.Hypertension. 2011 May;57(5):873-9. doi: 10.1161/HYPERTENSIONAHA.108.120246. Epub 2011 Mar 14. Hypertension. 2011. PMID: 21403087 Free PMC article. Review. No abstract available.
-
Heterogeneity of tight junctions in the thick ascending limb.Ann N Y Acad Sci. 2017 Oct;1405(1):5-15. doi: 10.1111/nyas.13400. Epub 2017 Jun 19. Ann N Y Acad Sci. 2017. PMID: 28628195 Review.
-
Regulation of thick ascending limb transport: role of nitric oxide.Am J Physiol Renal Physiol. 2006 Jun;290(6):F1279-84. doi: 10.1152/ajprenal.00465.2005. Am J Physiol Renal Physiol. 2006. PMID: 16682483 Review.
-
The renal medullary endothelin system in control of sodium and water excretion and systemic blood pressure.Curr Opin Nephrol Hypertens. 2006 Jan;15(1):34-40. doi: 10.1097/01.mnh.0000186852.15889.1a. Curr Opin Nephrol Hypertens. 2006. PMID: 16340664 Review.
Cited by
-
Dahl Salt-Resistant Rat Is Protected against Hypertension during Diet-Induced Obesity.Nutrients. 2022 Sep 16;14(18):3843. doi: 10.3390/nu14183843. Nutrients. 2022. PMID: 36145220 Free PMC article.
-
Interpreting the efficacy enhancement mechanism of Chinese medicine processing from a biopharmaceutic perspective.Chin Med. 2024 Jan 18;19(1):14. doi: 10.1186/s13020-024-00887-0. Chin Med. 2024. PMID: 38238801 Free PMC article. Review.
-
Impact of angiotensin II-mediated stimulation of sodium transporters in the nephron assessed by computational modeling.Am J Physiol Renal Physiol. 2019 Dec 1;317(6):F1656-F1668. doi: 10.1152/ajprenal.00335.2019. Epub 2019 Oct 28. Am J Physiol Renal Physiol. 2019. PMID: 31657247 Free PMC article.
-
IRBITs, signaling molecules of great functional diversity.Pflugers Arch. 2025 Aug;477(8):1007-1036. doi: 10.1007/s00424-025-03095-3. Epub 2025 May 30. Pflugers Arch. 2025. PMID: 40445299 Review.
-
Pathophysiology of Diuretic Resistance and Its Implications for the Management of Chronic Heart Failure.Hypertension. 2020 Oct;76(4):1045-1054. doi: 10.1161/HYPERTENSIONAHA.120.15205. Epub 2020 Aug 24. Hypertension. 2020. PMID: 32829662 Free PMC article. Review.
References
-
- Abassi ZA, Ellahham S, Winaver J, Hoffman A. The intrarenal endothelin system and hypertension. News Physiol Sci 16: 152–156, 2001. - PubMed
-
- Abu-Soud HM, Yoho LL, Stuehr DJ. Calmodulin controls neuronal nitric-oxide synthase by a dual mechanism. Activation of intra- and interdomain electron transfer. J Biol Chem 269: 32047–32050, 1994. - PubMed
-
- Ackermann D, Gresko N, Carrel M, Loffing-Cueni D, Habermehl D, Gomez-Sanchez C, Rossier BC, Loffing J. In vivo nuclear translocation of mineralocorticoid and glucocorticoid receptors in rat kidney: differential effect of corticosteroids along the distal tubule. Am J Physiol Renal Physiol 299: F1473–F1485, 2010. doi:10.1152/ajprenal.00437.2010. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

