Response Gene to Complement 32 Maintains Blood Pressure Homeostasis by Regulating α-Adrenergic Receptor Expression
- PMID: 30355157
- PMCID: PMC6214659
- DOI: 10.1161/CIRCRESAHA.118.313266
Response Gene to Complement 32 Maintains Blood Pressure Homeostasis by Regulating α-Adrenergic Receptor Expression
Abstract
Rationale: Hypertension prevalence is much higher among children and adolescents with low birth weight and greater postnatal weight gain than in individuals with normal birth weight. However, the cause and molecular mechanisms underlying this complication remain largely unknown. Our previous studies have shown that RGC-32 (response gene to complement 32)-deficient (RGC-32-/-) mice are born significantly smaller but grow faster than their WT (wild type) controls, which allows adult RGC-32-/- mice to attain body weights similar to those of control mice.
Objective: The objective of this study is to determine whether RGC-32-/- mice develop hypertension, and if so, to elucidate the underlying mechanisms.
Methods and results: By using a radiotelemetry system, we found that RGC-32-/- mice exhibit higher mean arterial pressure than WT mice (101±4 versus 119±5 mm Hg), which enabled us to use RGC-32-/- mice to study the mechanisms underlying low birth weight-related hypertension. The increased blood pressure in RGC-32-/- mice was associated with increased vascular tone and decreased distensibility of small resistance arteries. The increased vascular tone was because of an increase in the relative contribution of sympathetic versus parasympathetic activity and was linked to increased expression of AT1R (angiotensin II type I receptor) and α1-AdR (α1-adrenergic receptor) in arterial smooth muscles. Mechanistically, RGC-32 regulated AT1R gene transcription by interacting with Sp1 (specificity protein 1) transcription factor and further blocking its binding to the AT1R promoter, leading to suppression of AT1R expression. The attenuation of AT1R leads to reduction in α1-AdR expression, which was critical for the balance of sympathetic versus parasympathetic control of vascular tone. Of importance, downregulation of RGC-32 in arterial smooth muscles was also associated with low birth weight and hypertension in humans.
Conclusions: Our results indicate that RGC-32 is a novel protein factor vital for maintaining blood pressure homeostasis, especially in individuals with low birth weight.
Keywords: angiotensin II; arterial pressure; arteries; blood pressure; hypertension; low birth weight.
Figures


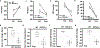
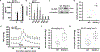



References
-
- Staessen JA, Wang J, Bianchi G, Birkenhäger WH. Essential hypertension. Lancet. 2003; 361:1629–1641. - PubMed
-
- Bruno RM, Faconti L, Taddei S, Ghiadoni L. Birth weight and arterial hypertension. Curr Opin Cardiol. 2015; 30:398–402. - PubMed
-
- Mitchell P, Liew G, Rochtchina E, Wang JJ, Robaei D, Cheung N, Wong TY. Evidence of arteriolar narrowing in low-birth-weight children. Circulation. 2008; 118:518–524. - PubMed
-
- Gishti O, Jaddoe VW, Duijts L, Steegers E, Reiss I, Hofman A, Wong TY, Ikram MK, Gaillard R. Impact of birth parameters and early life growth patterns on retinal microvascular structure in children: The Generation R Study. J Hypertens. 2015; 33:1429–1437. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

