Role of Thrombospondin-1 in Mechanotransduction and Development of Thoracic Aortic Aneurysm in Mouse and Humans
- PMID: 30355232
- PMCID: PMC6211815
- DOI: 10.1161/CIRCRESAHA.118.313105
Role of Thrombospondin-1 in Mechanotransduction and Development of Thoracic Aortic Aneurysm in Mouse and Humans
Erratum in
-
Correction to: Role of Thrombospondin-1 in Mechanotransduction and Development of Thoracic Aortic Aneurysm in Mouse and Humans.Circ Res. 2020 Aug 28;127(6):e142. doi: 10.1161/RES.0000000000000433. Epub 2020 Aug 27. Circ Res. 2020. PMID: 32853092 No abstract available.
Abstract
Rationale: Abnormal mechanosensing of smooth muscle cells (SMCs) resulting from the defective elastin-contractile units has been suggested to drive the formation of thoracic aortic aneurysms; however, the precise molecular mechanism has not been elucidated.
Objective: The aim of this study was to identify the crucial mediator(s) involved in abnormal mechanosensing and propagation of biochemical signals during the aneurysm formation and to establish a basis for a novel therapeutic strategy.
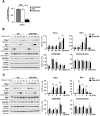
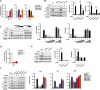
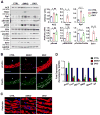
Methods and results: We used a mouse model of postnatal ascending aortic aneurysms ( Fbln4SMKO; termed SMKO [SMC-specific knockout]), in which deletion of Fbln4 (fibulin-4) leads to disruption of the elastin-contractile units caused by a loss of elastic lamina-SMC connections. In this mouse, upregulation of Egr1 (early growth response 1) and angiotensin-converting enzyme leads to activation of Ang II (angiotensin II) signaling. Here, we showed that the matricellular protein, Thbs1 (thrombospondin-1), was highly upregulated in SMKO ascending aortas and in human thoracic aortic aneurysms. Thbs1 was induced by mechanical stretch and Ang II in SMCs, for which Egr1 was required, and reduction of Fbln4 sensitized the cells to these stimuli and led to higher expression of Egr1 and Thbs1. Deletion of Thbs1 in SMKO mice prevented the aneurysm formation in ≈80% of DKO (SMKO;Thbs1 knockout) animals and suppressed Ssh1 (slingshot-1) and cofilin dephosphorylation, leading to the formation of normal actin filaments. Furthermore, elastic lamina-SMC connections were restored in DKO aortas, and mechanical testing showed that structural and material properties of DKO aortas were markedly improved.
Conclusions: Thbs1 is a critical component of mechanotransduction, as well as a modulator of elastic fiber organization. Maladaptive upregulation of Thbs1 results in disruption of elastin-contractile units and dysregulation of actin cytoskeletal remodeling, contributing to the development of ascending aortic aneurysms in vivo. Thbs1 may serve as a potential therapeutic target for treating thoracic aortic aneurysms.
Keywords: angiotensin II; aortic aneurysm, thoracic; elastic tissue; extracellular matrix; humans.
Figures



References
-
- Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of tgf-beta activation contributes to pathogenesis in marfan syndrome. Nat Genet. 2003;33:407–411. - PubMed
-
- Mizuguchi T, Collod-Beroud G, Akiyama T, Abifadel M, Harada N, Morisaki T, Allard D, Varret M, Claustres M, Morisaki H, Ihara M, Kinoshita A, Yoshiura K, Junien C, Kajii T, Jondeau G, Ohta T, Kishino T, Furukawa Y, Nakamura Y, Niikawa N, Boileau C, Matsumoto N. Heterozygous tgfbr2 mutations in marfan syndrome. Nat Genet. 2004;36:855–860. - PMC - PubMed
-
- Loeys BL, Chen J, Neptune ER, Judge DP, Podowski M, Holm T, Meyers J, Leitch CC, Katsanis N, Sharifi N, Xu FL, Myers LA, Spevak PJ, Cameron DE, De Backer J, Hellemans J, Chen Y, Davis EC, Webb CL, Kress W, Coucke P, Rifkin DB, De Paepe AM, Dietz HC. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in tgfbr1 or tgfbr2. Nat Genet. 2005;37:275–281. - PubMed
-
- Maki JM, Rasanen J, Tikkanen H, Sormunen R, Makikallio K, Kivirikko KI, Soininen R. Inactivation of the lysyl oxidase gene lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation. 2002;106:2503–2509. - PubMed
-
- Dasouki M, Markova D, Garola R, Sasaki T, Charbonneau NL, Sakai LY, Chu ML. Compound heterozygous mutations in fibulin-4 causing neonatal lethal pulmonary artery occlusion, aortic aneurysm, arachnodactyly, and mild cutis laxa. Am J Med Genet A. 2007;143A:2635–2641. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

