Clinical and economical impacts of guideline implementation by the pharmaceutical care unit for high cost medications in a referral teaching hospital
- PMID: 30355286
- PMCID: PMC6201544
- DOI: 10.1186/s12913-018-3627-3
Clinical and economical impacts of guideline implementation by the pharmaceutical care unit for high cost medications in a referral teaching hospital
Abstract
Background: Irrational drug use is a global health challenge in all healthcare settings, such as hospitals. This study evaluated the impact of an intervention by the pharmaceutical care unit on the use pattern of high-value medications and their direct costs in a referral hospital.
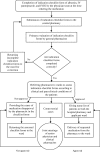
Methods: This interventional, prospective study was carried out in clinical wards of Namazi Hospital (Shiraz University of Medical Sciences) during six months from May 2015 to October 2015. Clinical pharmacists completed the checklists for albumin, intravenous (IV) pantoprazole, and IV immune globulin (IVIG), as three high-cost medications. When ordering these medications, the physicians were asked to complete the checklists. Then, trained pharmacists examined the checklists, based on the clinical and paraclinical conditions.
Results: The total number of administered medications and their relative cost decreased by 50.76% through guideline implementation; the difference was significant (P < 0.001). In addition, the direct cost of albumin and IV pantoprazole significantly decreased (55.8% and 83.92%, respectively). In contrast, the direct cost of IVIG increased by 40.9%. After guideline implementation, the monthly direct cost of all three medications decreased by $77,720 (55.88%). The all-cause in-hospital mortality rate did not change significantly due to the intervention. The median length of hospital stay was six and seven days, respectively in the pre- and post-intervention periods.
Conclusion: Based on the findings, implementation of guidelines by the pharmaceutical care unit caused a significant reduction in albumin and IV pantoprazole consumption and reduced their direct costs in a referral center in Iran.
Keywords: Albumin; Direct cost; Guideline implementation; Intravenous immune globulin; Intravenous pantoprazole; Pharmaceutical care unit.
Conflict of interest statement
Ethics approval and consent to participate
The Institutional Review Board and the Medical Ethics Committee of the Shiraz University of Medical Sciences approved the study and written informed consent form was received from patients or their family members to participate into the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Holloway K, Green T. Drug and therapeutics committees: a practical guide: World Health Organization; 2003. Available at: http://apps.who.int/medicinedocs/en/d/Js4882e/. Accessed 27 March 2017
MeSH terms
Substances
LinkOut - more resources
Full Text Sources