FUNDAMANT: an interventional 72-week phase 1 follow-up study of AADvac1, an active immunotherapy against tau protein pathology in Alzheimer's disease
- PMID: 30355322
- PMCID: PMC6201586
- DOI: 10.1186/s13195-018-0436-1
FUNDAMANT: an interventional 72-week phase 1 follow-up study of AADvac1, an active immunotherapy against tau protein pathology in Alzheimer's disease
Abstract
Background: Neurofibrillary pathology composed of tau protein is closely correlated with severity and phenotype of cognitive impairment in patients with Alzheimer's disease and non-Alzheimer's tauopathies. Targeting pathological tau proteins via immunotherapy is a promising strategy for disease-modifying treatment of Alzheimer's disease. Previously, we reported a 24-week phase 1 trial on the active vaccine AADvac1 against pathological tau protein; here, we present the results of a further 72 weeks of follow-up on those patients.
Methods: We did a phase 1, 72-week, open-label study of AADvac1 in patients with mild to moderate Alzheimer's disease who had completed the preceding phase 1 study. Patients who were previously treated with six doses of AADvac1 at monthly intervals received two booster doses at 24-week intervals. Patients who were previously treated with only three doses received another three doses at monthly intervals, and subsequently two boosters at 24-week intervals. The primary objective was the assessment of long-term safety of AADvac1 treatment. Secondary objectives included assessment of antibody titres, antibody isotype profile, capacity of the antibodies to bind to AD tau and AADvac1, development of titres of AADvac1-induced antibodies over time, and effect of booster doses; cognitive assessment via 11-item Alzheimer's Disease Assessment Scale cognitive assessment (ADAS-Cog), Category Fluency Test and Controlled Oral Word Association Test; assessment of brain atrophy via magnetic resonance imaging (MRI) volumetry; and assessment of lymphocyte populations via flow cytometry.
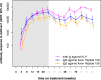
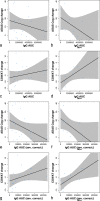
Results: The study was conducted between 18 March 2014 and 10 August 2016. Twenty-six patients who completed the previous study were enrolled. Five patients withdrew because of adverse events. One patient was withdrawn owing to noncompliance. The most common adverse events were injection site reactions (reported in 13 [50%] of vaccinated patients). No cases of meningoencephalitis or vasogenic oedema were observed. New micro-haemorrhages were observed only in one ApoE4 homozygote. All responders retained an immunoglobulin G (IgG) antibody response against the tau peptide component of AADvac1 over 6 months without administration, with titres regressing to a median 15.8% of titres attained after the initial six-dose vaccination regimen. Booster doses restored previous IgG levels. Hippocampal atrophy rate was lower in patients with high IgG levels; a similar relationship was observed in cognitive assessment.
Conclusions: AADvac1 displayed a benign safety profile. The evolution of IgG titres over vaccination-free periods warrants a more frequent booster dose regimen. The tendency towards slower atrophy in MRI evaluation and less of a decline in cognitive assessment in patients with high titres is encouraging. Further trials are required to expand the safety database and to establish proof of clinical efficacy of AADvac1.
Trial registration: The studies are registered with the EU Clinical Trials Register and ClinicalTrials.gov : the preceding first-in-human study under EudraCT 2012-003916-29 and NCT01850238 (registered on 9 May 2013) and the follow-up study under EudraCT 2013-004499-36 and NCT02031198 (registered 9 Jan 2014), respectively.
Keywords: Active immunotherapy; Alzheimer’s disease; Clinical trial; Immunotherapy; Neurofibrillary pathology; Tau; Vaccine.
Conflict of interest statement
Competing interests
Authors affiliated with AXON Neuroscience SE, AXON Neuroscience CRM Services SE and AXON Neuroscience R&D Services SE receive salary from the company. The investigators’ institutions have received payments on a per-patient per-visit basis. BW has received personal fees from AXON Neuroscience SE for advisory services.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

