A randomized, controlled study to evaluate the efficacy of intra-articular, autologous adipose tissue injections for the treatment of mild-to-moderate knee osteoarthritis compared to hyaluronic acid: a study protocol
- PMID: 30355323
- PMCID: PMC6201482
- DOI: 10.1186/s12891-018-2300-7
A randomized, controlled study to evaluate the efficacy of intra-articular, autologous adipose tissue injections for the treatment of mild-to-moderate knee osteoarthritis compared to hyaluronic acid: a study protocol
Abstract
Background: Osteoarthritis (OA) is a highly debilitating joint disease that causes progressive, irreversible damage to articular cartilage. OA takes a massive toll on society that has grown in recent decades, but no therapy has been shown to halt or reverse the progression of the disease. The critical need for better treatments and increased interest cellular therapies has spawned a new generation of "minimally manipulated" cell treatments. Autologous adipose tissue injections are among the most controversial of these new treatments. Despite a lack of clinical evidence, adipose tissue injections are often marketed as "stem cell" injections with wide-ranging regenerative benefits. The purpose of this study is to estimate the effect size of the treatment by comparing the efficacy of autologous fat to hyaluronic acid (HA). As a secondary aim, we will test for preliminary evidence of efficacy of autologous fat vs. HA.
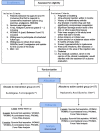
Methods: This is a prospective, single-center, parallel-group, randomized, controlled trial. Participants (n = 54) will receive either a single intra-articular, ultrasound-guided injection of autologous adipose tissue or a single intra-articular, ultrasound-guided injection of HA (1:1 ratio). Outcome data will be obtained at baseline, week-6 and month-6. The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain domain (WOMAC-A) will be used as the primary outcome measure. Secondary clinical outcome measures include WOMAC (full), clinical anchors (pain, function, and stiffness), and the 29-point Patient-Reported Outcomes Measurement Information System (PROMIS®) profile. We will also take synovial fluid samples and assess sway velocity using a force plate, as well as analyze excess/discard adipose tissue to gain a better understanding of how intra-articular adipose tissue injections influence the biochemical environment of the joint.
Discussion: Given the widespread use of intra-articular fat injections in the United States, it is critical that randomized, controlled human studies evaluating efficacy and biological activity be performed. This study is the first step in addressing this unmet need, but it is not without limitations. The most notable limitations of this study are its small size and lack of blinding, which predisposes the study to both investigator and participant bias.
Trial registration: NCT03242707 // HS-17-00365 // Registration Date (First Posted): August 8, 2018.
Keywords: ADSC; Adipose tissue; Fat MSC; Intra-articular injection; Knee; Lipogems; Osteoarthritis; Stem cell.
Conflict of interest statement
Ethics approval and consent to participate
This study was granted ethics approval by the University of Southern California Health Science IRB (IRB# HS-17-00365) on 10/9/2017 and USC Institutional Biosafety Committee (BUA-17-00026). All participants provided written informed consent to participate. A copy of informed consent document has been included (Additional file 4).
Consent for publication
N/A.
Competing interests
Ian A Jones has nothing to disclose.
Melissa Wilson has nothing to disclose.
Ryan Togashi has nothing to disclose.
Bo Han has nothing to disclose.
Austin K. Mircheff, has nothing to disclose.
C. Thomas Vangsness, Jr., is a shareholder and board member of CarthroniX.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Similar articles
-
Intra-articular injection of autologous adipose-derived stromal vascular fractions for knee osteoarthritis: a double-blind randomized self-controlled trial.Int Orthop. 2019 May;43(5):1123-1134. doi: 10.1007/s00264-018-4099-0. Epub 2018 Aug 14. Int Orthop. 2019. PMID: 30109404 Clinical Trial.
-
Autologous adipose tissue injection versus platelet-rich plasma (PRP) injection in the treatment of knee osteoarthritis: a randomized, controlled study - study protocol.BMC Musculoskelet Disord. 2020 May 20;21(1):314. doi: 10.1186/s12891-020-03345-8. BMC Musculoskelet Disord. 2020. PMID: 32434498 Free PMC article.
-
Ultrasound-guided intra-articular injection: efficacy of hyaluronic acid compared to glucocorticoid in the treatment of knee osteoarthritis.Minerva Med. 2019 Dec;110(6):515-523. doi: 10.23736/S0026-4806.19.06190-1. Minerva Med. 2019. PMID: 31965779
-
Intra-articular Injection of Autologous Adipose-Derived Stem Cells or Stromal Vascular Fractions: Are They Effective for Patients With Knee Osteoarthritis? A Systematic Review With Meta-analysis of Randomized Controlled Trials.Am J Sports Med. 2023 Mar;51(3):837-848. doi: 10.1177/03635465211053893. Epub 2022 Jan 12. Am J Sports Med. 2023. PMID: 35019764
-
Platelet-Rich Plasma Versus Hyaluronic Acid in the Treatment of Knee Osteoarthritis: A Meta-analysis of 26 Randomized Controlled Trials.Arthroscopy. 2021 Jan;37(1):309-325. doi: 10.1016/j.arthro.2020.07.011. Epub 2020 Jul 15. Arthroscopy. 2021. PMID: 32679294
Cited by
-
Minimally Manipulated Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis: A Systematic Review of Clinical Evidence.Stem Cells Int. 2019 Aug 14;2019:1735242. doi: 10.1155/2019/1735242. eCollection 2019. Stem Cells Int. 2019. PMID: 31485234 Free PMC article. Review.
-
Autologous cultured adipose derived mesenchymal stem cells combined with hyaluronic acid hydrogel in the treatment of discogenic low back pain: a study protocol for a phase II randomised controlled trial.BMJ Open. 2022 Oct 25;12(10):e063925. doi: 10.1136/bmjopen-2022-063925. BMJ Open. 2022. PMID: 36283750 Free PMC article.
-
Stem Cells in Autologous Microfragmented Adipose Tissue: Current Perspectives in Osteoarthritis Disease.Int J Mol Sci. 2021 Sep 22;22(19):10197. doi: 10.3390/ijms221910197. Int J Mol Sci. 2021. PMID: 34638538 Free PMC article. Review.
-
Regenerative Medicine: A Review of the Evolution of Autologous Chondrocyte Implantation (ACI) Therapy.Bioengineering (Basel). 2019 Mar 13;6(1):22. doi: 10.3390/bioengineering6010022. Bioengineering (Basel). 2019. PMID: 30871236 Free PMC article. Review.
-
A double-blinded, placebo-controlled, randomized study to evaluate the efficacy of perioperative dextromethorphan compared to placebo for the treatment of postoperative pain: a study protocol.Trials. 2023 Mar 29;24(1):238. doi: 10.1186/s13063-023-07240-0. Trials. 2023. PMID: 36991450 Free PMC article.
References
-
- Ethgen O, Kahler KH, Kong SX, Reginster J-Y, Wolfe F. The effect of health related quality of life on reported use of health care resources in patients with osteoarthritis and rheumatoid arthritis: a longitudinal analysis. J Rheumatol. 2002;29:1147–1155. - PubMed
-
- Kotlarz H, Gunnarsson CL, Fang H, Rizzo JA. Insurer and out-of-pocket costs of osteoarthritis in the US: evidence from national survey data. Arthritis Rheum. Wiley subscription services, Inc., A Wiley Company. 2009;60:3546–3553. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials