Expanded Interactome of the Intrinsically Disordered Protein Dss1
- PMID: 30355493
- PMCID: PMC6218214
- DOI: 10.1016/j.celrep.2018.09.080
Expanded Interactome of the Intrinsically Disordered Protein Dss1
Abstract
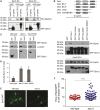
Dss1 (also known as Sem1) is a conserved, intrinsically disordered protein with a remarkably broad functional diversity. It is a proteasome subunit but also associates with the BRCA2, RPA, Csn12-Thp1, and TREX-2 complexes. Accordingly, Dss1 functions in protein degradation, DNA repair, transcription, and mRNA export. Here in Schizosaccharomyces pombe, we expand its interactome further to include eIF3, the COP9 signalosome, and the mitotic septins. Within its intrinsically disordered ensemble, Dss1 forms a transiently populated C-terminal helix that dynamically interacts with and shields a central binding region. The helix interfered with the interaction to ATP-citrate lyase but was required for septin binding, and in strains lacking Dss1, ATP-citrate lyase solubility was reduced and septin rings were more persistent. Thus, even weak, transient interactions within Dss1 may dynamically rewire its interactome.
Keywords: PCI domain; intrinsically disordered proteins; proteasome; protein degradation.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
DSS1/Sem1, a Multifunctional and Intrinsically Disordered Protein.Trends Biochem Sci. 2016 May;41(5):446-459. doi: 10.1016/j.tibs.2016.02.004. Epub 2016 Mar 1. Trends Biochem Sci. 2016. PMID: 26944332 Review.
-
Phosphorylation of Schizosaccharomyces pombe Dss1 mediates direct binding to the ubiquitin-ligase Dma1 in vitro.Protein Sci. 2023 Sep;32(9):e4733. doi: 10.1002/pro.4733. Protein Sci. 2023. PMID: 37463013 Free PMC article.
-
Dss1 is a 26S proteasome ubiquitin receptor.Mol Cell. 2014 Nov 6;56(3):453-461. doi: 10.1016/j.molcel.2014.09.008. Epub 2014 Oct 9. Mol Cell. 2014. PMID: 25306921 Free PMC article.
-
Fission yeast Dss1 associates with the proteasome and is required for efficient ubiquitin-dependent proteolysis.Biochem J. 2006 Jan 1;393(Pt 1):303-9. doi: 10.1042/BJ20051238. Biochem J. 2006. PMID: 16149916 Free PMC article.
-
Intrinsically disordered proteins and biomineralization.Matrix Biol. 2016 May-Jul;52-54:43-59. doi: 10.1016/j.matbio.2016.01.007. Epub 2016 Jan 22. Matrix Biol. 2016. PMID: 26807759 Free PMC article. Review.
Cited by
-
Deciphering the Alphabet of Disorder-Glu and Asp Act Differently on Local but Not Global Properties.Biomolecules. 2022 Oct 4;12(10):1426. doi: 10.3390/biom12101426. Biomolecules. 2022. PMID: 36291634 Free PMC article.
-
OB-fold Families of Genome Guardians: A Universal Theme Constructed From the Small β-barrel Building Block.Front Mol Biosci. 2022 Feb 11;9:784451. doi: 10.3389/fmolb.2022.784451. eCollection 2022. Front Mol Biosci. 2022. PMID: 35223988 Free PMC article. Review.
-
Distinct Functions of the Cap-Binding Complex in Stimulation of Nuclear mRNA Export.Mol Cell Biol. 2019 Apr 2;39(8):e00540-18. doi: 10.1128/MCB.00540-18. Print 2019 Apr 15. Mol Cell Biol. 2019. PMID: 30745412 Free PMC article.
-
The disordered PCI-binding human proteins CSNAP and DSS1 have diverged in structure and function.Protein Sci. 2021 Oct;30(10):2069-2082. doi: 10.1002/pro.4159. Epub 2021 Jul 26. Protein Sci. 2021. PMID: 34272906 Free PMC article.
-
Assessing Transcriptomic Responses to Oxidative Stress: Contrasting Wild-Type Arabidopsis Seedlings with dss1(I) and dss1(V) Gene Knockout Mutants.Int J Mol Sci. 2024 Jun 7;25(12):6291. doi: 10.3390/ijms25126291. Int J Mol Sci. 2024. PMID: 38927997 Free PMC article.
References
-
- Borcherds W., Theillet F.X., Katzer A., Finzel A., Mishall K.M., Powell A.T., Wu H., Manieri W., Dieterich C., Selenko P. Disorder and residual helicity alter p53-Mdm2 binding affinity and signaling in cells. Nat. Chem. Biol. 2014;10:1000–1002. - PubMed
-
- Bui J.M., Gsponer J. Phosphorylation of an intrinsically disordered segment in Ets1 shifts conformational sampling toward binding-competent substates. Structure. 2014;22:1196–1203. - PubMed
-
- Chypre M., Zaidi N., Smans K. ATP-citrate lyase: a mini-review. Biochem. Biophys. Res. Commun. 2012;422:1–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

