Asymmetric and Distant Effects of a Unilateral Lesion of the Primary Motor Cortex on the Bilateral Supplementary Motor Areas in Adult Macaque Monkeys
- PMID: 30355637
- PMCID: PMC6580657
- DOI: 10.1523/JNEUROSCI.0904-18.2018
Asymmetric and Distant Effects of a Unilateral Lesion of the Primary Motor Cortex on the Bilateral Supplementary Motor Areas in Adult Macaque Monkeys
Abstract
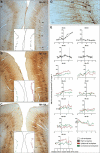
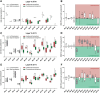
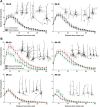
A restricted lesion of the hand area in the primary motor cortex (M1) leads to a deficit of contralesional manual dexterity, followed by an incomplete functional recovery, accompanied by plastic changes in M1 itself and in other cortical areas on both hemispheres. Using the marker SMI-32 specific to pyramidal neurons in cortical layers III and V, we investigated the impact of a focal unilateral M1 lesion (hand representation) on the rostral part (F6) and caudal part (F3) of the supplementary motor area (SMA) in both hemispheres in nine adult macaque monkeys compared with four intact control monkeys. The M1 lesion induced a consistent interhemispheric asymmetry in density of SMI-32-positive neurons in F3 layer V (statistically significant in 8 of 9 lesioned monkeys), highly correlated with the lesion volume and with the duration of functional recovery, but not with the extent of functional recovery itself. Such interhemispheric asymmetry was neither present in the intact monkeys, as expected, nor in F6 in all monkeys. In addition, the M1 lesion also impacted on the basal dendritic arborization of F3 layer V neurons. Neuronal density was clearly less affected by the M1 lesion in F3 layer III compared with layer V. We interpret the remote effect of M1 lesion onto the density of SMI-32-positive neurons and dendritic arborization in the SMAs bilaterally as the consequence of multiple factors, such as changes of connectivity, diaschisis and various mechanisms involved in cortical plasticity underlying the functional recovery from the M1 lesion.SIGNIFICANCE STATEMENT The motor system of macaque monkeys, in addition to be similarly organized as in humans, is a good candidate to study the impact of a focal lesion of the main contributor to voluntary movements, the primary motor cortex (M1), on non-primary motor cortical areas also involved in manual dexterity, both at behavioral and structural levels. Our results show that a unilateral permanent lesion of M1 hand area in nine monkeys affects the interhemispheric balance of the number of SMI-32-positive pyramidal neurons in the cortical layer V of the supplementary motor area, in a way strongly correlated to the lesion volume and duration of the incomplete functional recovery.
Keywords: cortical lesion; diaschisis; manual dexterity; motor cortex; nonhuman primate.
Copyright © 2018 the authors 0270-6474/18/3810644-13$15.00/0.
Figures


References
-
- Bashir S, Kaeser M, Wyss A, Hamadjida A, Liu Y, Bloch J, Brunet JF, Belhaj-Saif A, Rouiller EM (2012) Short-term effects of unilateral lesion of the primary motor cortex (M1) on ipsilesional hand dexterity in adult macaque monkeys. Brain Struct Funct 217:63–79. 10.1007/s00429-011-0327-8 - DOI - PMC - PubMed
-
- Beaud ML, Schmidlin E, Wannier T, Freund P, Bloch J, Mir A, Schwab ME, Rouiller EM (2008) Anti-nogo-A antibody treatment does not prevent cell body shrinkage in the motor cortex in adult monkeys subjected to unilateral cervical cord lesion. BMC Neurosci 9:5. 10.1186/1471-2202-9-5 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous
