Modifying Saccharomyces cerevisiae Adhesion Properties Regulates Yeast Ecosystem Dynamics
- PMID: 30355663
- PMCID: PMC6200983
- DOI: 10.1128/mSphere.00383-18
Modifying Saccharomyces cerevisiae Adhesion Properties Regulates Yeast Ecosystem Dynamics
Abstract
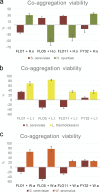
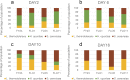
Physical contact between yeast species, in addition to better-understood and reported metabolic interactions, has recently been proposed to significantly impact the relative fitness of these species in cocultures. Such data have been generated by using membrane bioreactors, which physically separate two yeast species. However, doubts persist about the degree that the various membrane systems allow for continuous and complete metabolic contact, including the exchange of proteins. Here, we provide independent evidence for the importance of physical contact by using a genetic system to modify the degree of physical contact and, therefore, the degree of asexual intraspecies and interspecies adhesion in yeast. Such adhesion is controlled by a family of structurally related cell wall proteins encoded by the FLO gene family. As previously shown, the expression of specific members of the FLO gene family in Saccharomyces cerevisiae dramatically changes the coadhesion patterns between this yeast and other yeast species. Here, we use this differential aggregation mediated by FLO genes as a model to assess the impact of physical contact between different yeast species on the relative fitness of these species in simplified ecosystems. The identity of the FLO gene has a marked effect on the persistence of specific non-Saccharomyces yeasts over the course of extended growth periods in batch cultures. Remarkably, FLO1 and FLO5 expression often result in opposite outcomes. The data provide clear evidence for the role of physical contact in multispecies yeast ecosystems and suggest that FLO gene expression may be a major factor in such interactions.IMPORTANCE The impact of direct (physical) versus indirect (metabolic) interactions between different yeast species has attracted significant research interest in recent years. This is due to the growing interest in the use of multispecies consortia in bioprocesses of industrial relevance and the relevance of interspecies interactions in establishing stable synthetic ecosystems. Compartment bioreactors have traditionally been used in this regard but suffer from numerous limitations. Here, we provide independent evidence for the importance of physical contact by using a genetic system, based on the FLO gene family, to modify the degree of physical contact and, therefore, the degree of asexual intraspecies and interspecies adhesion in yeast. Our results show that interspecies contact significantly impacts population dynamics and the survival of individual species. Remarkably, different members of the FLO gene family often lead to very different population outcomes, further suggesting that FLO gene expression may be a major factor in such interactions.
Keywords: adhesion; cell-cell interaction; interspecies; yeast.
Copyright © 2018 Rossouw et al.
Figures




Similar articles
-
Co-Flocculation of Yeast Species, a New Mechanism to Govern Population Dynamics in Microbial Ecosystems.PLoS One. 2015 Aug 28;10(8):e0136249. doi: 10.1371/journal.pone.0136249. eCollection 2015. PLoS One. 2015. PMID: 26317200 Free PMC article.
-
Physical cell-cell contact elicits specific transcriptomic responses in wine yeast species.Microbiol Spectr. 2024 Aug 6;12(8):e0057223. doi: 10.1128/spectrum.00572-23. Epub 2024 Jul 16. Microbiol Spectr. 2024. PMID: 39012115 Free PMC article.
-
FLO gene-dependent phenotypes in industrial wine yeast strains.Appl Microbiol Biotechnol. 2010 Apr;86(3):931-45. doi: 10.1007/s00253-009-2381-1. Epub 2009 Dec 16. Appl Microbiol Biotechnol. 2010. PMID: 20013339
-
Flocculation protein structure and cell-cell adhesion mechanism in Saccharomyces cerevisiae.Biotechnol Lett. 2010 Nov;32(11):1571-85. doi: 10.1007/s10529-010-0352-3. Epub 2010 Jul 18. Biotechnol Lett. 2010. PMID: 20640875 Review.
-
Cell signals, cell contacts, and the organization of yeast communities.Eukaryot Cell. 2011 Apr;10(4):466-73. doi: 10.1128/EC.00313-10. Epub 2011 Feb 4. Eukaryot Cell. 2011. PMID: 21296916 Free PMC article. Review.
Cited by
-
Ecological interactions are a primary driver of population dynamics in wine yeast microbiota during fermentation.Sci Rep. 2020 Mar 18;10(1):4911. doi: 10.1038/s41598-020-61690-z. Sci Rep. 2020. PMID: 32188881 Free PMC article.
-
The influence of flocculation upon global gene transcription in a yeast CYC8 mutant.Microb Genom. 2024 Mar;10(3):001216. doi: 10.1099/mgen.0.001216. Microb Genom. 2024. PMID: 38529898 Free PMC article.
-
Understanding Wine through Yeast Interactions.Microorganisms. 2021 Jul 29;9(8):1620. doi: 10.3390/microorganisms9081620. Microorganisms. 2021. PMID: 34442699 Free PMC article. Review.
-
Protein Biosynthesis and Carbon Catabolite Repression Are Transcriptionally Upregulated in Saccharomyces cerevisiae by Extracellular Fractions From Several Wine Yeast Species.Microb Biotechnol. 2025 May;18(5):e70168. doi: 10.1111/1751-7915.70168. Microb Biotechnol. 2025. PMID: 40407676 Free PMC article.
-
The Third International Symposium on Fungal Stress - ISFUS.Fungal Biol. 2020 May;124(5):235-252. doi: 10.1016/j.funbio.2020.02.007. Epub 2020 Feb 24. Fungal Biol. 2020. PMID: 32389286 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
