Low-pH Endocytic Entry of the Porcine Alphaherpesvirus Pseudorabies Virus
- PMID: 30355685
- PMCID: PMC6321905
- DOI: 10.1128/JVI.01849-18
Low-pH Endocytic Entry of the Porcine Alphaherpesvirus Pseudorabies Virus
Abstract
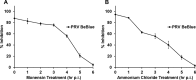
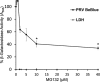
The alphaherpesvirus pseudorabies virus (PRV) is the causative agent of pseudorabies, a disease of great economic and welfare importance in swine. Other alphaherpesviruses, including herpes simplex virus (HSV), utilize low-pH-mediated endocytosis to enter a subset of cell types. We investigated whether PRV used this entry pathway in multiple laboratory model cell lines. Inhibition of receptor-mediated endocytosis by treatment with hypertonic medium prevented PRV entry. PRV entry into several cell lines, including porcine kidney (PK15) cells and African green monkey kidney (Vero) cells, was inhibited by noncytotoxic concentrations of the lysosomotropic agents ammonium chloride and monensin, which block the acidification of endosomes. Inactivation of virions by acid pretreatment is a hallmark of viruses that utilize a low-pH-mediated entry pathway. Exposure of PRV virions to pH 5.0 in the absence of host cell membranes reduced entry into PK15 and Vero cells by >80%. Together, these findings suggest that endocytosis followed by fusion with host membranes triggered by low endosomal pH is an important route of entry for PRV.IMPORTANCE PRV is a pathogen of great economic and animal welfare importance in many parts of the world. PRV causes neurological, respiratory, and reproductive disorders, often resulting in mortality of young and immunocompromised animals. Mortality, decreased production, and trade restrictions result in significant financial losses for the agricultural industry. Understanding the molecular mechanisms utilized by PRV to enter host cells is an important step in identifying novel strategies to prevent infection and spread. A thorough understanding of these mechanisms will contribute to a broader understanding of alphaherpesvirus entry. Here, we demonstrate PRV entry into multiple model cell lines via a low-pH endocytosis pathway. Together, these results provide a framework for elucidating the early events of the PRV replicative cycle.
Keywords: endocytosis; herpesviruses; low pH; pseudorabies virus; viral entry.
Copyright © 2019 American Society for Microbiology.
Figures




References
-
- Enquist LW. 1999. Life beyond eradication: veterinary viruses in basic science. Arch Virol Suppl 15:87–109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

