The NS1 Protein of Influenza A Virus Participates in Necroptosis by Interacting with MLKL and Increasing Its Oligomerization and Membrane Translocation
- PMID: 30355688
- PMCID: PMC6321931
- DOI: 10.1128/JVI.01835-18
The NS1 Protein of Influenza A Virus Participates in Necroptosis by Interacting with MLKL and Increasing Its Oligomerization and Membrane Translocation
Abstract
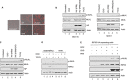
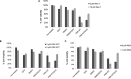
Elimination of infected cells by programmed cell death is a well-recognized host defense mechanism to control the spread of infection. In addition to apoptosis, necroptosis is also one of the mechanisms of cell death that can be activated by viral infection. Activation of necroptosis leads to the phosphorylation of mixed-lineage kinase domain-like protein (MLKL) by receptor-interacting protein kinase 3 (RIPK3) and results in MLKL oligomerization and membrane translocation, leading to membrane disruption and a loss of cellular ion homeostasis. It has recently been reported that influenza A virus (IAV) infection induces necroptosis. However, the underlying mechanism of the IAV-mediated necroptosis process, particularly the roles of IAV proteins in necroptosis, remains unexplored. Here, we report that IAV infection induces necroptosis in macrophages and epithelial cells. We demonstrate that the NS1 protein of IAV interacts with MLKL. Coiled-coil domain 2 of MLKL has a predominant role in mediating the MLKL interaction with NS1. The interaction of NS1 with MLKL increases MLKL oligomerization and membrane translocation. Moreover, the MLKL-NS1 interaction enhances MLKL-mediated NLRP3 inflammasome activation, leading to increased interleukin-1β (IL-1β) processing and secretion.IMPORTANCE Necroptosis is a programmed cell death that is inflammatory in nature owing to the release of danger-associated molecular patterns from the ruptured cell membrane. However, necroptosis also constitutes an important arm of host immune responses. Thus, a balanced inflammatory response determines the disease outcome. We report that the NS1 protein of IAV participates in necroptosis by interacting with MLKL, resulting in increased MLKL oligomerization and membrane translocation. These results reveal a novel function of the NS1 protein and the mechanism by which IAV induces necroptosis. Moreover, we show that this interaction enhances NLRP3 inflammasome activation and IL-1β processing and secretion. This information may contribute to a better understanding of the role of necroptosis in IAV-induced inflammation.
Keywords: MLKL; NS1 protein; inflammasome; influenza A virus; necroptosis.
Copyright © 2019 American Society for Microbiology.
Figures




Similar articles
-
Active MLKL triggers the NLRP3 inflammasome in a cell-intrinsic manner.Proc Natl Acad Sci U S A. 2017 Feb 7;114(6):E961-E969. doi: 10.1073/pnas.1613305114. Epub 2017 Jan 17. Proc Natl Acad Sci U S A. 2017. PMID: 28096356 Free PMC article.
-
MLKL-Driven Inflammasome Activation and Caspase-8 Mediate Inflammatory Cell Death in Influenza A Virus Infection.mBio. 2023 Apr 25;14(2):e0011023. doi: 10.1128/mbio.00110-23. Epub 2023 Feb 28. mBio. 2023. PMID: 36852999 Free PMC article.
-
Opposite Effects of Apoptotic and Necroptotic Cellular Pathways on Rotavirus Replication.J Virol. 2022 Jan 12;96(1):e0122221. doi: 10.1128/JVI.01222-21. Epub 2021 Oct 20. J Virol. 2022. PMID: 34668777 Free PMC article.
-
Viral-induced neuronal necroptosis: Detrimental to brain function and regulation by necroptosis inhibitors.Biochem Pharmacol. 2023 Jul;213:115591. doi: 10.1016/j.bcp.2023.115591. Epub 2023 May 16. Biochem Pharmacol. 2023. PMID: 37196683 Review.
-
Insane in the membrane: a structural perspective of MLKL function in necroptosis.Immunol Cell Biol. 2017 Feb;95(2):152-159. doi: 10.1038/icb.2016.125. Epub 2017 Jan 17. Immunol Cell Biol. 2017. PMID: 27999433 Review.
Cited by
-
A Not-So-Good Way to Die? Respiratory Syncytial Virus-induced Necroptotic Cell Death Promotes Inflammation and Type 2-mediated Pathology.Am J Respir Crit Care Med. 2020 Jun 1;201(11):1321-1323. doi: 10.1164/rccm.202003-0533ED. Am J Respir Crit Care Med. 2020. PMID: 32182121 Free PMC article. No abstract available.
-
From threat to cure: understanding of virus-induced cell death leads to highly immunogenic oncolytic influenza viruses.Cell Death Discov. 2020 Jun 11;6:48. doi: 10.1038/s41420-020-0284-1. eCollection 2020. Cell Death Discov. 2020. PMID: 32542113 Free PMC article. Review.
-
Molecular Events Involved in Influenza A Virus-Induced Cell Death.Front Microbiol. 2022 Jan 7;12:797789. doi: 10.3389/fmicb.2021.797789. eCollection 2021. Front Microbiol. 2022. PMID: 35069499 Free PMC article. Review.
-
Race between virus and inflammasomes: inhibition or escape, intervention and therapy.Front Cell Infect Microbiol. 2023 Jul 3;13:1173505. doi: 10.3389/fcimb.2023.1173505. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37465759 Free PMC article. Review.
-
Necroptosis in Pneumonia: Therapeutic Strategies and Future Perspectives.Viruses. 2024 Jan 7;16(1):94. doi: 10.3390/v16010094. Viruses. 2024. PMID: 38257794 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

