A MicroRNA Derived from Adenovirus Virus-Associated RNAII Promotes Virus Infection via Posttranscriptional Gene Silencing
- PMID: 30355689
- PMCID: PMC6321910
- DOI: 10.1128/JVI.01265-18
A MicroRNA Derived from Adenovirus Virus-Associated RNAII Promotes Virus Infection via Posttranscriptional Gene Silencing
Abstract
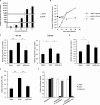
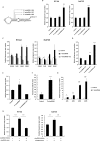
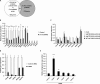
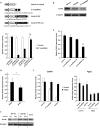
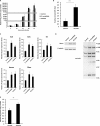
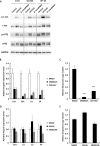
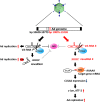
The adenovirus (Ad) serotype 5 genome encodes two noncoding small RNAs (virus-associated RNAs I and II [VA-RNAI and -II]), which are approximately 160-nucleotide (nt) RNAs transcribed by RNA polymerase III. It is well known that VA-RNAI supports Ad infection via the inhibition of double-stranded RNA-dependent protein kinase (PKR), which recognizes double-stranded RNA and acts as an antiviral system. Recent studies revealed that VA-RNAs are processed into VA-RNA-derived microRNAs (miRNAs) (mivaRNAI and -II); however, we and another group recently demonstrated that mivaRNAI does not promote Ad replication. On the other hand, the roles of VA-RNAII and mivaRNAII in Ad replication have remained to be clarified. In this study, we demonstrated mivaRNAII-mediated promotion of Ad replication. Transfection with chemically synthesized 3'-mivaRNAII-138, one of the most abundant forms of mivaRNAII, significantly enhanced Ad replication, while the other species of mivaRNAII did not. We identified 8 putative target genes of 3'-mivaRNAII-138 by microarray analysis and in silico analysis. Among the 8 candidates, knockdown of the cullin 4A (CUL4A) gene, which encodes a component of the ubiquitin ligase complex, most significantly enhanced Ad replication. CUL4A expression was significantly suppressed by 3'-mivaRNAII-138 via posttranscriptional gene silencing, indicating that CUL4A is a target gene of 3'-mivaRNAII-138 and mivaRNAII functions as a viral miRNA promoting Ad infection. It has been reported that CUL4A is involved in degradation of c-Jun, which acts as a transcription factor in the Jun-N-terminal kinase (JNK) signaling cascade. Treatment with JNK inhibitors dramatically suppressed Ad replication, suggesting that mivaRNAII-mediated downregulation of CUL4A enhanced JNK signaling and thereby promoted Ad infection.IMPORTANCE Several types of viruses encode viral miRNAs which regulate host and/or viral gene expression via posttranscriptional gene silencing, leading to efficient viral infection. Adenovirus (Ad) expresses miRNAs derived from VA-RNAs (mivaRNAI and -II); however, recent studies have revealed that processing of VA-RNAI into mivaRNAI inhibits Ad replication. Conversely, we demonstrate here that mivaRNAII significantly promotes Ad replication and that mivaRNAII-mediated suppression of CUL4A expression via posttranscriptional gene silencing induces accumulation of c-Jun, leading to promotion of Ad infection. These results exhibited the significance of VA-RNAII for supporting Ad infection through a mechanism complementary to that of VA-RNAI. These observations could provide important clues toward a new perspective on host-virus interaction. Moreover, Ad is widely used as a basic framework for viral vectors and oncolytic viruses. Our findings will help to regulate Ad infection and will promote the development of novel Ad vectors and oncolytic Ad.
Keywords: JNK signaling; adenoviruses; cullin 4A; microRNA; posttranscriptional gene silencing.
Copyright © 2019 American Society for Microbiology.
Figures
Similar articles
-
[Development of Novel Genetically Engineered Adenoviruses Based on Functional Analyses of Adenovirus-encoded Small RNAs].Yakugaku Zasshi. 2016;136(11):1509-1515. doi: 10.1248/yakushi.16-00170. Yakugaku Zasshi. 2016. PMID: 27803482 Review. Japanese.
-
MicroRNA miR-27 Inhibits Adenovirus Infection by Suppressing the Expression of SNAP25 and TXN2.J Virol. 2017 May 26;91(12):e00159-17. doi: 10.1128/JVI.00159-17. Print 2017 Jun 15. J Virol. 2017. PMID: 28356525 Free PMC article.
-
Small RNA sequence analysis of adenovirus VA RNA-derived miRNAs reveals an unexpected serotype-specific difference in structure and abundance.PLoS One. 2014 Aug 21;9(8):e105746. doi: 10.1371/journal.pone.0105746. eCollection 2014. PLoS One. 2014. PMID: 25144466 Free PMC article.
-
Identification of RISC-associated adenoviral microRNAs, a subset of their direct targets, and global changes in the targetome upon lytic adenovirus 5 infection.J Virol. 2015 Feb;89(3):1608-27. doi: 10.1128/JVI.02336-14. Epub 2014 Nov 19. J Virol. 2015. PMID: 25410853 Free PMC article.
-
Adenovirus VA RNA: An essential pro-viral non-coding RNA.Virus Res. 2016 Jan 2;212:39-52. doi: 10.1016/j.virusres.2015.06.018. Epub 2015 Jun 25. Virus Res. 2016. PMID: 26116898 Review.
Cited by
-
Global profiling of megalocytivirus-induced proteins in tongue sole (Cynoglossus semilaevis) spleen identifies cellular processes essential to viral infection.Dev Comp Immunol. 2019 Mar;92:150-159. doi: 10.1016/j.dci.2018.11.006. Epub 2018 Nov 11. Dev Comp Immunol. 2019. PMID: 30428365 Free PMC article.
-
MicroRNAs: Small but Key Players in Viral Infections and Immune Responses to Viral Pathogens.Biology (Basel). 2023 Oct 14;12(10):1334. doi: 10.3390/biology12101334. Biology (Basel). 2023. PMID: 37887044 Free PMC article. Review.
-
Quantitative Virus-Associated RNA Detection to Monitor Oncolytic Adenovirus Replication.Int J Mol Sci. 2024 Jun 14;25(12):6551. doi: 10.3390/ijms25126551. Int J Mol Sci. 2024. PMID: 38928259 Free PMC article.
-
Synthesis, Structure, and Function of Human Adenovirus Small Non-Coding RNAs.Viruses. 2020 Oct 19;12(10):1182. doi: 10.3390/v12101182. Viruses. 2020. PMID: 33086737 Free PMC article. Review.
-
Unleashing the Full Potential of Oncolytic Adenoviruses against Cancer by Applying RNA Interference: The Force Awakens.Cells. 2018 Nov 23;7(12):228. doi: 10.3390/cells7120228. Cells. 2018. PMID: 30477117 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

