Biogenesis of Extracellular Vesicles during Herpes Simplex Virus 1 Infection: Role of the CD63 Tetraspanin
- PMID: 30355691
- PMCID: PMC6321934
- DOI: 10.1128/JVI.01850-18
Biogenesis of Extracellular Vesicles during Herpes Simplex Virus 1 Infection: Role of the CD63 Tetraspanin
Abstract
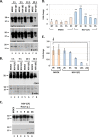
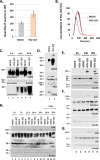
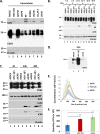
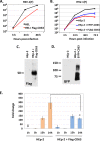
Herpes simplex virus 1 (HSV-1) infections afflict more than 80% of the population worldwide. The virus primarily infects mucoepithelial cells and establishes latent reservoirs in neurons in sensory ganglia. Frequent reactivation has been linked to severe diseases, especially in immunocompromised individuals. Earlier, we reported that viral and host factors are packaged in extracellular vesicles (EVs) and delivered to uninfected cells, where they activate antiviral responses and restrict virus infection. Here, we interrogated the effect of HSV-1 infection on EV biogenesis. We found that HSV-1 infection causes a decrease in the amount of intracellular CD63 protein with a concomitant increase in extracellular CD63. This observation correlates with our previous finding that infected cells release more CD63-positive EVs than uninfected cells. The stimulation of CD63 exocytosis requires virus replication. CD63 is a member of the tetraspanin family of proteins that traffics between the plasma membrane and endosomal compartments and has a role in sorting cargo into the EVs. Previously, we reported that in cells depleted of CD63, HSV-1 virus yields increased, and here we provide data showing that in cells overexpressing CD63, HSV-1 virus yields decreased. Taken together, our data indicate that CD63 negatively impacts HSV-1 infection and that the CD63-positive EVs could control the dissemination of the virus in the host. Perhaps EV release by HSV-1-infected cells is a mechanism that controls virus dissemination.IMPORTANCE Intercellular communication, especially in neurons, largely relies on EVs, and modulation of EVs is known to impact physiological processes. Here, we present evidence that HSV-1 infection causes major alterations in the biogenesis of EVs, including an increase in their number and an increase in the CD63-positive population of EVs. These alterations result in an enrichment of the milieu of infection with EVs carrying signatures from infected cells. In addition to changes in the origin and type, EVs released by infected cells have differences in cargo, as they carry viral and host factors determined by the virus. The tetraspanin CD63 negatively impacts the infection, as demonstrated by CD63-knockdown and overexpression assays. A proposed mechanism involves the activation of antiviral responses in cells receiving CD63-positive EVs released by infected cells. Overall, HSV-1 causes major alterations in EVs that could contribute to HSV-1 persistence and pathogenesis.
Keywords: CD63; CD81; HSV-1; biogenesis of extracellular vesicles; exocytosis; extracellular vesicles; tetraspanins.
Copyright © 2019 American Society for Microbiology.
Figures
Similar articles
-
Diverse Populations of Extracellular Vesicles with Opposite Functions during Herpes Simplex Virus 1 Infection.J Virol. 2021 Feb 24;95(6):e02357-20. doi: 10.1128/JVI.02357-20. Print 2021 Feb 24. J Virol. 2021. PMID: 33361424 Free PMC article.
-
Extracellular Vesicles Released by Herpes Simplex Virus 1-Infected Cells Block Virus Replication in Recipient Cells in a STING-Dependent Manner.J Virol. 2018 Aug 29;92(18):e01102-18. doi: 10.1128/JVI.01102-18. Print 2018 Sep 15. J Virol. 2018. PMID: 29976662 Free PMC article.
-
HSV-1 virions and related particles: biogenesis and implications in the infection.J Virol. 2025 Mar 18;99(3):e0107624. doi: 10.1128/jvi.01076-24. Epub 2025 Feb 3. J Virol. 2025. PMID: 39898651 Free PMC article. Review.
-
Tracing the STING exocytosis pathway during herpes viruses infection.mBio. 2024 Apr 10;15(4):e0037324. doi: 10.1128/mbio.00373-24. Epub 2024 Mar 12. mBio. 2024. PMID: 38470056 Free PMC article.
-
Extracellular vesicles during Herpes Simplex Virus type 1 infection: an inquire.Virol J. 2016 Apr 5;13:63. doi: 10.1186/s12985-016-0518-2. Virol J. 2016. PMID: 27048572 Free PMC article. Review.
Cited by
-
The CD63 homologs, Tsp42Ee and Tsp42Eg, restrict endocytosis and promote neurotransmission through differential regulation of synaptic vesicle pools.Front Cell Neurosci. 2022 Aug 22;16:957232. doi: 10.3389/fncel.2022.957232. eCollection 2022. Front Cell Neurosci. 2022. PMID: 36072568 Free PMC article.
-
The ICP0 Protein of Herpes Simplex Virus 1 (HSV-1) Downregulates Major Autophagy Adaptor Proteins Sequestosome 1 and Optineurin during the Early Stages of HSV-1 Infection.J Virol. 2019 Oct 15;93(21):e01258-19. doi: 10.1128/JVI.01258-19. Print 2019 Nov 1. J Virol. 2019. PMID: 31375597 Free PMC article.
-
A Defective Viral Particle Approach to COVID-19.Cells. 2022 Jan 17;11(2):302. doi: 10.3390/cells11020302. Cells. 2022. PMID: 35053418 Free PMC article. Review.
-
Complexities of JC Polyomavirus Receptor-Dependent and -Independent Mechanisms of Infection.Viruses. 2022 May 24;14(6):1130. doi: 10.3390/v14061130. Viruses. 2022. PMID: 35746603 Free PMC article. Review.
-
Xenogenous implanted dental follicle stem cells promote periodontal regeneration through inducing the N2 phenotype of neutrophils.Stem Cell Res Ther. 2024 Aug 26;15(1):270. doi: 10.1186/s13287-024-03882-2. Stem Cell Res Ther. 2024. PMID: 39183362 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

