Encapsidation of Viral RNA in Picornavirales: Studies on Cowpea Mosaic Virus Demonstrate Dependence on Viral Replication
- PMID: 30355698
- PMCID: PMC6321914
- DOI: 10.1128/JVI.01520-18
Encapsidation of Viral RNA in Picornavirales: Studies on Cowpea Mosaic Virus Demonstrate Dependence on Viral Replication
Abstract
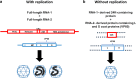
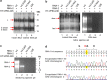
To elucidate the linkage between replication and encapsidation in Picornavirales, we have taken advantage of the bipartite nature of a plant-infecting member of this order, cowpea mosaic virus (CPMV), to decouple the two processes. RNA-free virus-like particles (empty virus-like particles [eVLPs]) can be generated by transiently coexpressing the RNA-2-encoded coat protein precursor (VP60) with the RNA-1-encoded 24,000-molecular-weight (24K) protease, in the absence of the replication machinery (K. Saunders, F. Sainsbury, and G. P. Lomonossoff, Virology 393:329-337, 2009, https://doi.org/10.1016/j.virol.2009.08.023). We have made use of the ability to produce assembled capsids of CPMV in the absence of replication to examine the putative linkage between RNA replication and packaging in the Picornavirales We have created a series of mutant RNA-1 and RNA-2 molecules and have assessed the effects of the mutations on both the replication and packaging of the viral RNAs. We demonstrate that mutations that affect replication have a concomitant impact on encapsidation and that RNA-1-mediated replication is required for encapsidation of both RNA-1 and RNA-2. This close coupling between replication and encapsidation provides a means for the specific packaging of viral RNAs. Moreover, we demonstrate that this feature of CPMV can be used to specifically encapsidate custom RNA by placing a sequence of choice between the RNA-2 sequences required for replication.IMPORTANCE The mechanism whereby members of the order Picornavirales specifically package their genomic RNAs is poorly understood. Research with monopartite members of the order, such as poliovirus, indicated that packaging is linked to replication, although the presence of "packaging signals" along the length of the viral RNA has also been suggested. Thanks to the bipartite nature of the CPMV genome, which allows the manipulation of RNA-1 without modifying RNA-2, we show here that this specificity is due to a functional link between the two processes of viral replication and encapsidation. This has important implications for our understanding of the fundamental molecular biology of Picornavirales and opens the door to novel research and therapeutic applications in the field of custom RNA packaging and delivery technologies.
Keywords: Picornavirales; RNA packaging; RNA virus; cowpea mosaic virus; plant viruses; viral encapsidation; viral replication.
Copyright © 2019 Kruse et al.
Figures








References
-
- Shakeel S, Westerhuis BM, Domanska A, Koning RI, Matadeen R, Koster AJ, Bakker AQ, Beaumont T, Wolthers KC, Butcher SJ. 2016. Multiple capsid-stabilizing interactions revealed in a high-resolution structure of an emerging picornavirus causing neonatal sepsis. Nat Commun 7:11387. doi: 10.1038/ncomms11387. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

