Correlation of HBcrAg with Intrahepatic Hepatitis B Virus Total DNA and Covalently Closed Circular DNA in HBeAg-Positive Chronic Hepatitis B Patients
- PMID: 30355757
- PMCID: PMC6322451
- DOI: 10.1128/JCM.01303-18
Correlation of HBcrAg with Intrahepatic Hepatitis B Virus Total DNA and Covalently Closed Circular DNA in HBeAg-Positive Chronic Hepatitis B Patients
Abstract
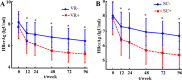
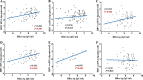
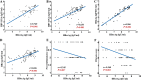
The purpose of this study was to explore the correlations of serum hepatitis B core-related antigen (HBcrAg) with intrahepatic Hepatitis B virus (HBV) covalently closed circular DNA (cccDNA) and HBV total DNA in hepatitis B e antigen (HBeAg)-positive chronic hepatitis B (CHB) patients. Serum HBcrAg and other parameters, including HBV DNA, HBV RNA, HBeAg, hepatitis B surface antigen (HBsAg), hepatitis B core antibody (anti-HBc), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were quantitatively measured at baseline and follow-up time points. Intrahepatic HBV cccDNA and total DNA were quantitatively detected at baseline and 96 weeks. Grading of liver necroinflammation and staging of hepatic fibrosis were assessed at baseline and 96 weeks. Correlations between serum HBcrAg and other parameters were analyzed by Pearson's correlation analysis. The results showed that pretreatment HBcrAg correlated significantly with HBV total DNA levels (r = 0.328, P = 0.003) in 82 CHB patients, and, after removing three outliers, with intrahepatic HBV cccDNA (r = 0.323, P = 0.004; n = 79). Serum HBcrAg correlated better with HBV cccDNA in patients with lower levels of serum HBV DNA (stratified by 7 log IU/ml of HBV DNA; r = 0.656, P = 0.003 versus r = -0.02, P = 0.866). Significant inverse correlations were found between HBcrAg and grade of liver necroinflammation (r = -0.245, P = 0.037), stage of hepatic fibrosis (r = -0.360, P = 0.002) at baseline. Serum HBcrAg presented significant correlation with intrahepatic HBV cccDNA in patients with HBeAg seroconversion at 96 weeks (r = 0.622, P = 0.006). The decrease in HBcrAg showed significant correlation with the decrease in HBV cccDNA after 96-week NA therapy (r = 0.282, P = 0.043). Serum HBcrAg also correlated significantly with other serum markers at baseline and 96 weeks of NA therapy. In conclusion, baseline HBcrAg and its decreased value were significantly correlated with the corresponding intrahepatic HBV cccDNA.
Keywords: HBV RNA; chronic hepatitis B; covalently closed circular DNA; hepatitis B core-related antigen; nucleos(t)ide analogues.
Copyright © 2019 American Society for Microbiology.
Figures
Similar articles
-
The Role of Hepatitis B Core-Related Antigen.Genes (Basel). 2019 May 9;10(5):357. doi: 10.3390/genes10050357. Genes (Basel). 2019. PMID: 31075974 Free PMC article. Review.
-
Serum hepatitis B core-related antigen (HBcrAg) correlates with covalently closed circular DNA transcriptional activity in chronic hepatitis B patients.J Hepatol. 2019 Apr;70(4):615-625. doi: 10.1016/j.jhep.2018.11.030. Epub 2018 Dec 7. J Hepatol. 2019. PMID: 30529504
-
Serum HBV RNA correlated with intrahepatic cccDNA more strongly than other HBV markers during peg-interferon treatment.Virol J. 2021 Jan 6;18(1):4. doi: 10.1186/s12985-020-01471-2. Virol J. 2021. PMID: 33407619 Free PMC article. Clinical Trial.
-
Serum Hepatitis B Virus DNA, RNA, and HBsAg: Which Correlated Better with Intrahepatic Covalently Closed Circular DNA before and after Nucleos(t)ide Analogue Treatment?J Clin Microbiol. 2017 Oct;55(10):2972-2982. doi: 10.1128/JCM.00760-17. Epub 2017 Jul 26. J Clin Microbiol. 2017. PMID: 28747369 Free PMC article.
-
Review article: hepatitis B core-related antigen (HBcrAg): an emerging marker for chronic hepatitis B virus infection.Aliment Pharmacol Ther. 2018 Jan;47(1):43-54. doi: 10.1111/apt.14376. Epub 2017 Oct 16. Aliment Pharmacol Ther. 2018. PMID: 29035003 Review.
Cited by
-
The Role of Hepatitis B Core-Related Antigen.Genes (Basel). 2019 May 9;10(5):357. doi: 10.3390/genes10050357. Genes (Basel). 2019. PMID: 31075974 Free PMC article. Review.
-
Quantitative Measurement of Serum HBcrAg Can Be Used to Assess the Feasibility of Safe Discontinuation of Antiviral Therapy for Chronic Hepatitis B.Viruses. 2024 Mar 29;16(4):529. doi: 10.3390/v16040529. Viruses. 2024. PMID: 38675872 Free PMC article. Review.
-
Hepatitis B core-related antigen: A novel and promising surrogate biomarker to guide anti-hepatitis B virus therapy.Clin Mol Hepatol. 2023 Oct;29(4):851-868. doi: 10.3350/cmh.2022.0434. Epub 2023 Mar 9. Clin Mol Hepatol. 2023. PMID: 36891607 Free PMC article. Review.
-
Characteristics of HBV Novel Serum Markers across Distinct Phases in Treatment-Naïve Chronic HBV-Infected Patients.Dis Markers. 2022 Jul 14;2022:4133283. doi: 10.1155/2022/4133283. eCollection 2022. Dis Markers. 2022. PMID: 35872701 Free PMC article.
-
The Predictive Role of Hepatitis B Biomarkers on HBV Reactivation following Direct-Acting Antiviral Therapy in HBV/HCV Coinfected Patients.Viruses. 2022 Aug 18;14(8):1812. doi: 10.3390/v14081812. Viruses. 2022. PMID: 36016434 Free PMC article.
References
-
- Bourne EJ, Dienstag JL, Lopez VA, Sander TJ, Longlet JM, Hall JG, Kwiatkowski RW, Wright T, Lai CL, Condreay LD. 2007. Quantitative analysis of HBV cccDNA from clinical specimens: correlation with clinical and virological response during antiviral therapy. J Viral Hepat 14:55–63. doi:10.1111/j.1365-2893.2006.00775.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

