Antibiotics induce sustained dysregulation of intestinal T cell immunity by perturbing macrophage homeostasis
- PMID: 30355800
- PMCID: PMC6548564
- DOI: 10.1126/scitranslmed.aao4755
Antibiotics induce sustained dysregulation of intestinal T cell immunity by perturbing macrophage homeostasis
Abstract
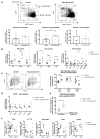
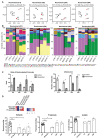
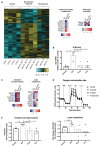
Macrophages in the healthy intestine are highly specialized and usually respond to the gut microbiota without provoking an inflammatory response. A breakdown in this tolerance leads to inflammatory bowel disease (IBD), but the mechanisms by which intestinal macrophages normally become conditioned to promote microbial tolerance are unclear. Strong epidemiological evidence linking disruption of the gut microbiota by antibiotic use early in life to IBD indicates an important role for the gut microbiota in modulating intestinal immunity. Here, we show that antibiotic use causes intestinal macrophages to become hyperresponsive to bacterial stimulation, producing excess inflammatory cytokines. Re-exposure of antibiotic-treated mice to conventional microbiota induced a long-term, macrophage-dependent increase in inflammatory T helper 1 (TH1) responses in the colon and sustained dysbiosis. The consequences of this dysregulated macrophage activity for T cell function were demonstrated by increased susceptibility to infections requiring TH17 and TH2 responses for clearance (bacterial Citrobacter rodentium and helminth Trichuris muris infections), corresponding with increased inflammation. Short-chain fatty acids (SCFAs) were depleted during antibiotic administration; supplementation of antibiotics with the SCFA butyrate restored the characteristic hyporesponsiveness of intestinal macrophages and prevented T cell dysfunction. Butyrate altered the metabolic behavior of macrophages to increase oxidative phosphorylation and also promoted alternative macrophage activation. In summary, the gut microbiota is essential to maintain macrophage-dependent intestinal immune homeostasis, mediated by SCFA-dependent pathways. Oral antibiotics disrupt this process to promote sustained T cell-mediated dysfunction and increased susceptibility to infections, highlighting important implications of repeated broad-spectrum antibiotic use.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures




Comment in
-
Tame or inflame: gut reactions to pro- and antibiotics.Nat Rev Immunol. 2018 Dec;18(12):728-729. doi: 10.1038/s41577-018-0093-4. Nat Rev Immunol. 2018. PMID: 30425346 No abstract available.
References
-
- Denning TL, Wang Y-C, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin-17–producing T cell responses. Nat Immunol. 2007;8:1086–1094. - PubMed
-
- Bain CC, Scott CL, Uronen-Hansson H, Gudjonsson S, Jansson O, Grip O, Guilliams M, Malissen B, Agace WW, Mowat AM. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 2013;6:498–510. - PMC - PubMed
-
- Hadis U, Wahl B, Schulz O, Hardtke-Wolenski M, Schippers A, Wagner N, Müller W, Sparwasser T, Förster R, Pabst O. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity. 2011;34:237–246. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 100974/WT_/Wellcome Trust/United Kingdom
- MR/K021095/1/MRC_/Medical Research Council/United Kingdom
- BBS/E/F/00044409/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/R003114/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MR/M00242X/1/MRC_/Medical Research Council/United Kingdom
- 206206/WT_/Wellcome Trust/United Kingdom
- BBS/E/F/000PR10356/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 203128/WT_/Wellcome Trust/United Kingdom
- BBS/E/F/000PR10353/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/J004529/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 097820/Z/11/B/WT_/Wellcome Trust/United Kingdom
- MR/N023625/1/MRC_/Medical Research Council/United Kingdom
- T32 DK007632/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

