The Intrinsic and Extrinsic Implications of RANKL/RANK Signaling in Osteosarcoma: From Tumor Initiation to Lung Metastases
- PMID: 30355966
- PMCID: PMC6265790
- DOI: 10.3390/cancers10110398
The Intrinsic and Extrinsic Implications of RANKL/RANK Signaling in Osteosarcoma: From Tumor Initiation to Lung Metastases
Abstract
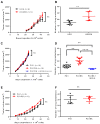
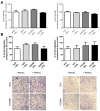
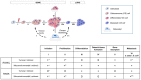
Background: Osteosarcoma is the most frequent form of malignant pediatric bone tumor. Despite the current therapeutic arsenal, patient life-expectancy remains low if metastases are detected at the time of diagnosis, justifying research into better knowledge at all stages of osteosarcoma ontogenesis and identification of new therapeutic targets. Receptor Activator of Nuclear factor κB (RANK)expression has been reported in osteosarcoma cells, raising the question of Receptor Activator of Nuclear factor κB Ligand (RANKL)/RANK signaling implications in these tumor cells (intrinsic), in addition to previously reported implications through osteoclast activation in the tumor microenvironment (extrinsic). Methods: Based on in vitro and in vivo experimentations using human and mouse osteosarcoma cell lines, the consequences on the main cellular processes of RANK expression in osteosarcoma cells were analyzed. Results: The results revealed that RANK expression had no impact on cell proliferation and tumor growth, but stimulated cellular differentiation and, in an immune-compromised environment, increased the number of lung metastases. The analysis of RANKL, RANK and osteoprotegerin (OPG) expressions in biopsies of a cohort of patients revealed that while RANK expression in osteosarcoma cells was not significantly different between patients with or without metastases at the time of diagnosis, the OPG/RANK ratio decreased significantly. Conclusion: Altogether, these results are in favor of RANKL-RANK signaling inhibition as an adjuvant for the treatment of osteosarcoma.
Keywords: RANKL/RANK; T-lymphocyte; bone; metastases; osteosarcoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Osteoprotegerin activates osteosarcoma cells that co-express RANK and RANKL.Exp Cell Res. 2015 Oct 15;338(1):32-8. doi: 10.1016/j.yexcr.2015.08.001. Epub 2015 Aug 5. Exp Cell Res. 2015. PMID: 26254896
-
Receptor activator of nuclear factor-kappaB ligand and osteoprotegerin: potential implications for the pathogenesis and treatment of malignant bone diseases.Cancer. 2001 Aug 1;92(3):460-70. doi: 10.1002/1097-0142(20010801)92:3<460::aid-cncr1344>3.0.co;2-d. Cancer. 2001. PMID: 11505389 Review.
-
Receptor activator of nuclear factor-kappaB ligand (RANKL) directly modulates the gene expression profile of RANK-positive Saos-2 human osteosarcoma cells.Oncol Rep. 2007 Dec;18(6):1365-71. Oncol Rep. 2007. PMID: 17982618
-
Association of RANKL and EGFR gene expression with bone metastases in patients with metastatic non-small cell lung cancer.Front Oncol. 2023 May 5;13:1145001. doi: 10.3389/fonc.2023.1145001. eCollection 2023. Front Oncol. 2023. PMID: 37213294 Free PMC article.
-
Receptor activator of nuclear factor-kappa B ligand (RANKL) stimulates bone-associated tumors through functional RANK expressed on bone-associated cancer cells?Histol Histopathol. 2009 Feb;24(2):235-42. doi: 10.14670/HH-24.235. Histol Histopathol. 2009. PMID: 19085839 Review.
Cited by
-
Update on Tenosynovial Giant Cell Tumor, an Inflammatory Arthritis With Neoplastic Features.Front Immunol. 2022 Feb 21;13:820046. doi: 10.3389/fimmu.2022.820046. eCollection 2022. Front Immunol. 2022. PMID: 35265077 Free PMC article.
-
High expression of ID1 facilitates metastasis in human osteosarcoma by regulating the sensitivity of anoikis via PI3K/AKT depended suppression of the intrinsic apoptotic signaling pathway.Am J Transl Res. 2019 Apr 15;11(4):2117-2139. eCollection 2019. Am J Transl Res. 2019. PMID: 31105823 Free PMC article.
-
The Osteosarcoma Microenvironment: A Complex But Targetable Ecosystem.Cells. 2020 Apr 15;9(4):976. doi: 10.3390/cells9040976. Cells. 2020. PMID: 32326444 Free PMC article. Review.
-
Multifunctional nanomaterials via cell cuproptosis and oxidative stress for treating osteosarcoma and OS-induced bone destruction.Mater Today Bio. 2024 Feb 15;25:100996. doi: 10.1016/j.mtbio.2024.100996. eCollection 2024 Apr. Mater Today Bio. 2024. PMID: 38420143 Free PMC article.
-
Association of Genetic Polymorphisms in TNFRSF11 with the Progression of Genetic Susceptibility to Gastric Cancer.J Oncol. 2020 Jun 22;2020:4103264. doi: 10.1155/2020/4103264. eCollection 2020. J Oncol. 2020. PMID: 32655638 Free PMC article.
References
-
- Heymann D. Bone Cancer. Academic Press; San Diego, CA, USA: 2010. Preface; p. ix.
-
- Nataraj V., Rastogi S., Khan S.A., Sharma M.C., Agarwala S., Vishnubhatla S., Bakhshi S. Prognosticating metastatic osteosarcoma treated with uniform chemotherapy protocol without high dose methotrexate and delayed metastasectomy: A single center experience of 102 patients. Clin. Transl. Oncol. 2016;18:937–944. doi: 10.1007/s12094-015-1467-8. - DOI - PubMed
LinkOut - more resources
Full Text Sources

