The Low Molecular Weight Heparin Tinzaparin Attenuates Platelet Activation in Terms of Metastatic Niche Formation by Coagulation-Dependent and Independent Pathways
- PMID: 30356007
- PMCID: PMC6278400
- DOI: 10.3390/molecules23112753
The Low Molecular Weight Heparin Tinzaparin Attenuates Platelet Activation in Terms of Metastatic Niche Formation by Coagulation-Dependent and Independent Pathways
Abstract
An intimate interplay with platelets is an initial key issue for tumor cells in terms of hematogenous metastasis. Tumor cells activate platelets by different pathways and receive, upon forming a platelet cloak, protection from immune surveillance and support in metastatic niche creation. Therapeutic intervention with this early interaction is promising to antagonize the whole metastatic cascade. Here we aimed to investigate the capability of low molecular weight heparin (LMWH), unfractionated heparin (UFH), and a non-anticoagulant heparin derivative or FXa inhibitor fondaparinux to interfere with platelet activation by tumor cells. Coagulation-dependent and independent pathways of platelet activation by three tumor cell lines, and interference therewith were analyzed by fluorigenic thrombin formation assay, platelet aggregometry, ATP and VEGF release and endothelial tube formation assay. LMWH and UFH were found to repress various routes of platelet activation, reflected by attenuated endothelial tube formation. This confirms the duality of anti-coagulative and anti-adhesive properties of heparin. While non-anticoagulative heparin (RO-heparin) depressed platelets' ATP and VEGF release by contact inhibition sufficiently, fondaparinux just attenuated tissue factor mediated thrombin generation. Concluding, these data suggest that LMWH as a guideline-based drug for anticoagulative strategies in oncology is promising to provide additional benefit for interference with metastatic activities.
Keywords: VEGF; heparin; metastatic niche; platelet; thrombin; tumor.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsor had no role in the design of the study; in the collection, analyses, or interpretation of data; in writing of the manuscript, and in the decision to publish the results.
Figures
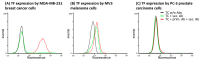
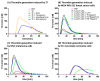

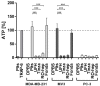

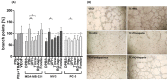
References
-
- Kakkar A.K., Levine M.N., Kadziola Z., Lemoine N.R., Low V., Patel H.K., Rustin G., Thomas M., Quigley M., Williamson R.C.N. Low molecular weight heparin, therapy with dalteparin, and survival in advanced cancer: The fragmin advanced malignancy outcome study (FAMOUS) J. Clin. Oncol. 2004;22:1944–1948. doi: 10.1200/JCO.2004.10.002. - DOI - PubMed
-
- Klerk C.P.W., Smorenburg S.M., Otten H.-M., Lensing A.W.A., Prins M.H., Piovella F., Prandoni P., Bos M.M.E.M., Richel D.J., van Tienhoven G., et al. The effect of low molecular weight heparin on survival in patients with advanced malignancy. J. Clin. Oncol. 2005;23:2130–2135. doi: 10.1200/JCO.2005.03.134. - DOI - PubMed
-
- Macbeth F., Noble S., Evans J., Ahmed S., Cohen D., Hood K., Knoyle D., Linnane S., Longo M., Moore B., et al. Randomized Phase III Trial of Standard Therapy Plus Low Molecular Weight Heparin in Patients With Lung Cancer: FRAGMATIC Trial. J. Clin. Oncol. 2016;34:488–494. doi: 10.1200/JCO.2015.64.0268. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

