Amplification of the Melanocortin-1 Receptor in Nephrotic Syndrome Identifies a Target for Podocyte Cytoskeleton Stabilization
- PMID: 30356069
- PMCID: PMC6200758
- DOI: 10.1038/s41598-018-34004-7
Amplification of the Melanocortin-1 Receptor in Nephrotic Syndrome Identifies a Target for Podocyte Cytoskeleton Stabilization
Abstract
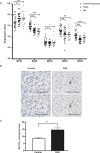
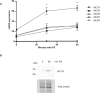
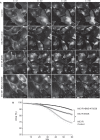
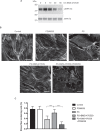
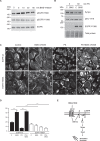
The melanocortin-1 receptor (MC1R) in podocytes has been suggested as the mediator of the ACTH renoprotective effect in patients with nephrotic syndrome with the mechanism of action beeing stabilization of the podocyte actin cytoskeleton. To understand how melanocortin receptors are regulated in nephrotic syndrome and how they are involved in restoration of filtration barrier function, melanocortin receptor expression was evaluated in patients and a rat model of nephrotic syndrome in combination with cell culture analysis. Phosphoproteomics was applied and identified MC1R pathways confirmed using biochemical analysis. We found that glomerular MC1R expression was increased in nephrotic syndrome, both in humans and in a rat model. A MC1R agonist protected podocytes from protamine sulfate induced stress fiber loss with the top ranked phoshoproteomic MC1R activated pathway beeing actin cytoskeleton signaling. Actin stabilization through the MC1R consisted of ERK1/2 dependent phosphorylation and inactivation of EGFR signaling with stabilization of synaptopodin and stressfibers in podocytes. These results further explain how patients with nephrotic syndrome show responsiveness to MC1R receptor activation by decreasing EGFR signaling and as a consequence restore filtration barrier function by stabilizing the podocyte actin cytoskeleton.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

