Reprogramming mechanisms influence the maturation of hematopoietic progenitors from human pluripotent stem cells
- PMID: 30356076
- PMCID: PMC6200746
- DOI: 10.1038/s41419-018-1124-6
Reprogramming mechanisms influence the maturation of hematopoietic progenitors from human pluripotent stem cells
Abstract
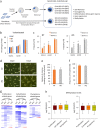
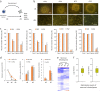
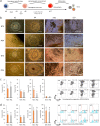
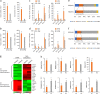
Somatic cell nuclear transfer (SCNT) or the forced expression of transcription factors can be used to generate autologous pluripotent stem cells (PSCs). Although transcriptomic and epigenomic comparisons of isogenic human NT-embryonic stem cells (NT-ESCs) and induced PSCs (iPSCs) in the undifferentiated state have been reported, their functional similarities and differentiation potentials have not been fully elucidated. Our study showed that NT-ESCs and iPSCs derived from the same donors generally displayed similar in vitro commitment capacity toward three germ layer lineages as well as proliferative activity and clonogenic capacity. However, the maturation capacity of NT-ESC-derived hematopoietic progenitors was significantly greater than the corresponding capacity of isogenic iPSC-derived progenitors. Additionally, donor-dependent variations in hematopoietic specification and commitment capacity were observed. Transcriptome and methylome analyses in undifferentiated NT-ESCs and iPSCs revealed a set of genes that may influence variations in hematopoietic commitment and maturation between PSC lines derived using different reprogramming methods. Here, we suggest that genetically identical iPSCs and NT-ESCs could be functionally unequal due to differential transcription and methylation levels acquired during reprogramming. Our proof-of-concept study indicates that reprogramming mechanisms and genetic background could contribute to diverse functionalities between PSCs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

