Angiogenic and Osteogenic Synergy of Human Mesenchymal Stem Cells and Human Umbilical Vein Endothelial Cells Cocultured on a Nanomatrix
- PMID: 30356078
- PMCID: PMC6200728
- DOI: 10.1038/s41598-018-34033-2
Angiogenic and Osteogenic Synergy of Human Mesenchymal Stem Cells and Human Umbilical Vein Endothelial Cells Cocultured on a Nanomatrix
Abstract
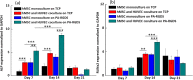
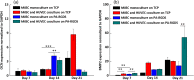
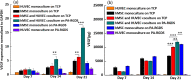
To date, bone tissue regeneration strategies lack an approach that effectively provides an osteogenic and angiogenic environment conducive to bone growth. In the current study, we evaluated the osteogenic and angiogenic response of human mesenchymal stem cells (hMSCs) and green fluorescent protein-expressing human umbilical vein endothelial cells (GFP-HUVECs) cocultured on a self-assembled, peptide amphiphile nanomatrix functionalized with the cell adhesive ligand RGDS (PA-RGDS). Analysis of alkaline phosphatase activity, von Kossa staining, Alizarin Red quantification, and osteogenic gene expression, indicates a significant synergistic effect between the PA-RGDS nanomatrix and coculture that promoted hMSC osteogenesis. In addition, coculturing on PA-RGDS resulted in enhanced HUVEC network formation and upregulated vascular endothelial growth factor gene and protein expression. Though PA-RGDS and coculturing hMSCs with HUVECs were each previously reported to individually enhance hMSC osteogenesis, this study is the first to demonstrate a synergistic promotion of HUVEC angiogenesis and hMSC osteogenesis by integrating coculturing with the PA-RGDS nanomatrix. We believe that using the combination of hMSC/HUVEC coculture and PA-RGDS substrate is an efficient method for promoting osteogenesis and angiogenesis, which has immense potential as an efficacious, engineered platform for bone tissue regeneration.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Parfitt, A. In Physiology and pharmacology of bone 1–65 (Springer, 1993).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

