Targeted killing of TNFR2-expressing tumor cells and Tregs by TNFR2 antagonistic antibodies in advanced Sézary syndrome
- PMID: 30356161
- PMCID: PMC6756055
- DOI: 10.1038/s41375-018-0292-9
Targeted killing of TNFR2-expressing tumor cells and Tregs by TNFR2 antagonistic antibodies in advanced Sézary syndrome
Abstract
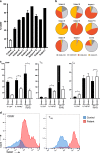
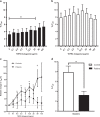
Sézary syndrome (SS) is a rare form of cutaneous T-cell lymphoma often refractory to treatment. SS is defined as adenopathy, erythroderma with high numbers of atypical T cells. This offers an opportunity for new interventions and perhaps antibody-based therapeutic by virtue of its high expression of the TNFR2 oncogene on the tumor cells and on T-regulatory cells (Tregs). Potent human-directed TNFR2 antagonistic antibodies have been created that preferentially target the TNFR2 oncogene and tumor-infiltrating TNFR2+ Tregs. Here we test the therapeutic potential of TNFR2 antagonists on freshly isolated lymphocytes from patients with Stage IVA SS and from healthy controls. SS patients were on a variety of end-stage multi-drug therapies. Baseline burden Treg/T effector (Teff) ratios and the responsiveness of tumor and infiltrating Tregs to TNFR2 antibody killing was studied. We show dose-escalating concentrations of a dominant TNFR2 antagonistic antibody killed TNFR2+ SS tumor cells and thus restored CD26- subpopulations of lymphocyte cell numbers to normal. The abundant TNFR2+ Tregs of SS subjects are also killed with TNFR2 antagonism. Beneficial and rapid expansion of Teff was observed. The combination of Treg inhibition and Teff expansion brought the high Treg/Teff ratio to normal. Our findings suggest a marked responsiveness of SS tumor cells and Tregs, to targeting with TNFR2 antagonistic antibodies. These results show TNFR2 antibodies are potent and efficacious in vitro.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Swerdlow S. H., Campo E., Pileri S. A., Harris N. L., Stein H., Siebert R., Advani R., Ghielmini M., Salles G. A., Zelenetz A. D., Jaffe E. S. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi: 10.1182/blood-2016-01-643569. - DOI - PMC - PubMed
-
- Teng MW, Ritchie DS, Neeson P, Smyth MJ. Biology and clinical observations of regulatory T cells in cancer immunology. Curr Top Microbiol Immunol. 2011;344:61–95. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

