A comprehensive in silico analysis for identification of therapeutic epitopes in HPV16, 18, 31 and 45 oncoproteins
- PMID: 30356257
- PMCID: PMC6200245
- DOI: 10.1371/journal.pone.0205933
A comprehensive in silico analysis for identification of therapeutic epitopes in HPV16, 18, 31 and 45 oncoproteins
Abstract
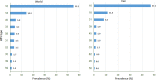
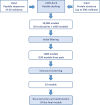
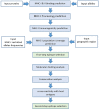
Human papillomaviruses (HPVs) are a group of circular double-stranded DNA viruses, showing severe tropism to mucosal tissues. A subset of HPVs, especially HPV16 and 18, are the primary etiological cause for several epithelial cell malignancies, causing about 5.2% of all cancers worldwide. Due to the high prevalence and mortality, HPV-associated cancers have remained as a significant health problem in human society, making an urgent need to develop an effective therapeutic vaccine against them. Achieving this goal is primarily dependent on the identification of efficient tumor-associated epitopes, inducing a robust cell-mediated immune response. Previous information has shown that E5, E6, and E7 early proteins are responsible for the induction and maintenance of HPV-associated cancers. Therefore, the prediction of major histocompatibility complex (MHC) class I T cell epitopes of HPV16, 18, 31 and 45 oncoproteins was targeted in this study. For this purpose, a two-step plan was designed to identify the most probable CD8+ T cell epitopes. In the first step, MHC-I and II binding, MHC-I processing, MHC-I population coverage and MHC-I immunogenicity prediction analyses, and in the second step, MHC-I and II protein-peptide docking, epitope conservation, and cross-reactivity with host antigens' analyses were carried out successively by different tools. Finally, we introduced five probable CD8+ T cell epitopes for each oncoprotein of the HPV genotypes (60 epitopes in total), which obtained better scores by an integrated approach. These predicted epitopes are valuable candidates for in vitro or in vivo therapeutic vaccine studies against the HPV-associated cancers. Additionally, this two-step plan that each step includes several analyses to find appropriate epitopes provides a rational basis for DNA- or peptide-based vaccine development.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

