Natural history of postnatal rhesus cytomegalovirus shedding by dams and acquisition by infant rhesus monkeys
- PMID: 30356332
- PMCID: PMC6200253
- DOI: 10.1371/journal.pone.0206330
Natural history of postnatal rhesus cytomegalovirus shedding by dams and acquisition by infant rhesus monkeys
Abstract
Background: Human infants frequently acquire human cytomegalovirus (HCMV) through breastfeeding, resulting in persistent high-level viral shedding in saliva and urine and infectivity to others, including pregnant women. Thus, vaccination to interrupt postnatal HCMV transmission is an attractive strategy to prevent HCMV spread and congenital infection. Rhesus CMV (RhCMV) in nonhuman primates is a valuable model for the study of immune strategies to prevent CMV transmission. Although rhesus monkeys typically acquire RhCMV before 1 year of age, the timing and mode of natural infant RhCMV transmission remain unknown.
Methods: We followed 5 RhCMV-seropositive dams and their infants from birth until weaning, approximately 6 months later. RhCMV DNA levels in plasma, breast milk, saliva, and urine were measured every 2 weeks by quantitative PCR. RhCMV-specific T cell responses in peripheral blood and breast milk were measured by interferon gamma ELISpot assays. Serum IgG antibody levels were measured by ELISA.
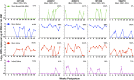
Results: Four of five postpartum RhCMV-seropositive mothers had intermittent, low-level RhCMV shedding in breast milk, whereas all had high-magnitude RhCMV shedding in saliva and urine. The kinetics of maternal blood RhCMV-specific T cell responses and viral shedding in urine and saliva did not strongly associate, though dams with consistently high systemic RhCMV-specific T cell responses tended to have undetectable RhCMV shedding in breast milk. All RhCMV-exposed infants had intermittent, low-level RhCMV shedding in saliva during the lactation period, with minimal systemic RhCMV-specific T cell responses.
Conclusions: Despite exposure to RhCMV shedding in breast milk and other maternal fluids, postnatal mother-to-child RhCMV transmission appears to be less efficient than that of HCMV. A greater understanding of the determinants of RhCMV transmission and its usefulness as a model of HCMV mucosal acquisition may provide insight into strategies to prevent HCMV infections in humans.
Conflict of interest statement
S.R.P. and A.K. provide consulting services to Pfizer Inc. for their preclinical HCMV vaccine program and associated animal models. S.R.P. also provides consulting services to Moderna Therapeutics for their HCMV vaccine program. S.G. receives research funding and consultancy fees from Merck and research funding from VBI Vaccines Inc. related to HCMV vaccine development, and research funding from Meridian Bioscience related to HCMV diagnostic development. The other authors declare no conflicts of interest. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

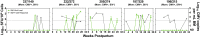
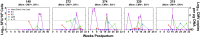

References
-
- Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2006;43(9):1143–51. Epub 2006/10/10. 10.1086/508173 . - DOI - PubMed
-
- Adler SP. The molecular epidemiology of cytomegalovirus transmission among children attending a day care center. The Journal of infectious diseases. 1985;152(4):760–8. Epub 1985/10/01. . - PubMed
-
- Hutto C, Ricks R, Garvie M, Pass RF. Epidemiology of cytomegalovirus infections in young children: day care vs. home care. Pediatric infectious disease. 1985;4(2):149–52. Epub 1985/03/01. . - PubMed
-
- Jones LA, Duke-Duncan PM, Yeager AS. Cytomegaloviral infections in infant-toddler centers: centers for the developmentally delayed versus regular day care. The Journal of infectious diseases. 1985;151(5):953–5. Epub 1985/05/01. . - PubMed

