Combined Treatment with Zinc Aspartate and Intravenous Immunoglobulins (IVIGs) Ameliorates Experimental Autoimmune Encephalomyelitis (EAE)
- PMID: 30356433
- PMCID: PMC6178489
- DOI: 10.1155/2018/5982169
Combined Treatment with Zinc Aspartate and Intravenous Immunoglobulins (IVIGs) Ameliorates Experimental Autoimmune Encephalomyelitis (EAE)
Abstract
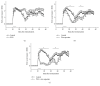
Intravenous immunoglobulins (IVIGs) are widely used in replacement therapy of primary and secondary immunodeficiency disorders and in approved autoimmune indications. In addition, IVIG application is used off-label for treatment of other autoimmune diseases, e.g., multiple sclerosis (MS), an inflammatory autoimmune disorder with a clear T cell-mediated immune pathogenesis. The trace element zinc is shown to play a regulatory role in the maintenance of immune functions. Changes of zinc homeostasis affect both the innate and the adaptive immune system. On one hand, therapeutic zinc supplementation can normalize impaired immune functions due to zinc deficiency. On the other hand, therapeutic zinc supplementation is under consideration as a possible option to treat T cell-mediated autoimmune diseases. The aim of the present study was to investigate the influence of IVIG (Octagam®), zinc aspartate (Unizink®), and the combined application of both preparations in the experimental autoimmune encephalomyelitis (EAE), the animal model of MS. Therapeutic intraperitoneal application of zinc aspartate significantly diminished clinical signs during the relapsing-remitting phase of EAE in SJL/J mice. In contrast, IVIG given in a therapeutic manner did not influence the course of EAE. Interestingly, the combined application of both, IVIG and zinc aspartate, significantly reduced the severity of the disease during the acute and the relapsing-remitting phase of the EAE. Our data suggest that the combination of IVIG and zinc aspartate may have beneficial effects in autoimmune diseases, like MS. Further studies should verify the benefit of a controlled immunosuppressive therapy with IVIG and zinc for such diseases.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

