Why are muscles strong, and why do they require little energy in eccentric action?
- PMID: 30356622
- PMCID: PMC6189244
- DOI: 10.1016/j.jshs.2018.05.005
Why are muscles strong, and why do they require little energy in eccentric action?
Abstract
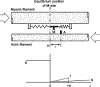
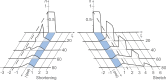
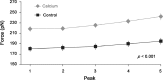
It is well acknowledged that muscles that are elongated while activated (i.e., eccentric muscle action) are stronger and require less energy (per unit of force) than muscles that are shortening (i.e., concentric contraction) or that remain at a constant length (i.e., isometric contraction). Although the cross-bridge theory of muscle contraction provides a good explanation for the increase in force in active muscle lengthening, it does not explain the residual increase in force following active lengthening (residual force enhancement), or except with additional assumptions, the reduced metabolic requirement of muscle during and following active stretch. Aside from the cross-bridge theory, 2 other primary explanations for the mechanical properties of actively stretched muscles have emerged: (1) the so-called sarcomere length nonuniformity theory and (2) the engagement of a passive structural element theory. In this article, these theories are discussed, and it is shown that the last of these-the engagement of a passive structural element in eccentric muscle action-offers a simple and complete explanation for many hitherto unexplained observations in actively lengthening muscle. Although by no means fully proven, the theory has great appeal for its simplicity and beauty, and even if over time it is shown to be wrong, it nevertheless forms a useful framework for direct hypothesis testing.
Keywords: Cross-bridge theory; Eccentric; Force–length relationship; Residual force enhancement; Titin.
Figures







References
-
- Hill AV. The heat of shortening and the dynamic constants of muscle. Proc R Soc Lond. 1938;126:136–195.
-
- Huxley AF. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. - PubMed
-
- Huxley AF. Liverpool University Press; Liverpool: 1980. Reflections on muscle.
-
- Herzog W, Schappacher G, Duvall M, Leonard TR, Herzog JA. Residual force enhancement following eccentric contractions: a new mechanism involving titin. Physiology. 2016;31:300–312. - PubMed
LinkOut - more resources
Full Text Sources

