Hyperphosphorylation of Tau Associates With Changes in Its Function Beyond Microtubule Stability
- PMID: 30356756
- PMCID: PMC6189415
- DOI: 10.3389/fncel.2018.00338
Hyperphosphorylation of Tau Associates With Changes in Its Function Beyond Microtubule Stability
Abstract
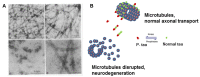
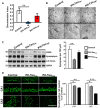
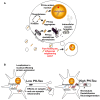
Tau is a neuronal microtubule associated protein whose main biological functions are to promote microtubule self-assembly by tubulin and to stabilize those already formed. Tau also plays an important role as an axonal microtubule protein. Tau is an amazing protein that plays a key role in cognitive processes, however, deposits of abnormal forms of tau are associated with several neurodegenerative diseases, including Alzheimer disease (AD), the most prevalent, and Chronic Traumatic Encephalopathy (CTE) and Traumatic Brain Injury (TBI), the most recently associated to abnormal tau. Tau post-translational modifications (PTMs) are responsible for its gain of toxic function. Alonso et al. (1996) were the first to show that the pathological tau isolated from AD brains has prion-like properties and can transfer its toxic function to the normal molecule. Furthermore, we reported that the pathological changes are associated with tau phosphorylation at Ser199 and 262 and Thr212 and 231. This pathological version of tau induces subcellular mislocalization in cultured cells and neurons, and translocates into the nucleus or accumulated in the perinuclear region of cells. We have generated a transgenic mouse model that expresses pathological human tau (PH-Tau) in neurons at two different concentrations (4% and 14% of the total endogenous tau). In this model, PH-Tau causes cognitive decline by at least two different mechanisms: one that involves the cytoskeleton with axonal disruption (at high concentration), and another in which the apparent neuronal morphology is not grossly affected, but the synaptic terminals are altered (at lower concentration). We will discuss the putative involvement of tau in proteostasis under these conditions. Understanding tau's biological activity on and off the microtubules will help shed light to the mechanism of neurodegeneration and of normal neuronal function.
Keywords: PH-tau; hyperphosphorylation; microtubules; neurodegeneration; propagation; tau.
Figures

References
-
- Alonso A. D., Cohen L. S., Morozova V. (2017). “The tau misfolding pathway to dementia,” in Protein Folding Disorders in the Central Nervous System, eds Ghiso J., Rostagno A. (New York, NY: World Scientific Publishing; ), 83–107.
-
- Alonso A. D., Grundke-Iqbal I., Barra H. S., Iqbal K. (1997). Abnormal phosphorylation of tau and the mechanism of Alzheimer neurofibrillary degeneration: sequestration of microtubule-associated proteins 1 and 2 and the disassembly of microtubules by the abnormal tau. Proc. Natl. Acad. Sci. U S A 94, 298–303. 10.1073/pnas.94.1.298 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

