Inflammatory Potential of Four Different Phases of Calcium Pyrophosphate Relies on NF-κB Activation and MAPK Pathways
- PMID: 30356764
- PMCID: PMC6189479
- DOI: 10.3389/fimmu.2018.02248
Inflammatory Potential of Four Different Phases of Calcium Pyrophosphate Relies on NF-κB Activation and MAPK Pathways
Abstract
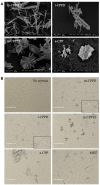
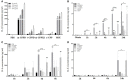
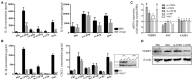
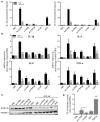
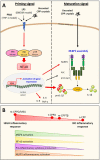
Background: Calcium pyrophosphate (CPP) microcrystal deposition is associated with wide clinical phenotypes, including acute and chronic arthritis, that are interleukin 1β (IL-1β)-driven. Two CPP microcrystals, namely monoclinic and triclinic CPP dihydrates (m- and t-CPPD), have been identified in human tissues in different proportions according to clinical features. m-CPP tetrahydrate beta (m-CPPTβ) and amorphous CPP (a-CPP) phases are considered as m- and t-CPPD crystal precursors in vitro. Objectives: We aimed to decipher the inflammatory properties of the three crystalline phases and one amorphous CPP phase and the intracellular pathways involved. Methods: The four synthesized CPP phases and monosodium urate crystals (MSU, as a control) were used in vitro to stimulate the human monocytic leukemia THP-1 cell line or bone marrow-derived macrophages (BMDM) isolated from WT or NLRP3 KO mice. The gene expression of pro- and anti-inflammatory cytokines was evaluated by quantitative PCR; IL-1β, IL-6 and IL-8 production by ELISA; and mitogen-activated protein kinase (MAPK) activation by immunoblot analysis. NF-κB activation was determined in THP-1 cells containing a reporter plasmid. In vivo, the inflammatory potential of CPP phases was assessed with the murine air pouch model via cell analysis and production of IL-1β and CXCL1 in the exudate. The role of NF-κB was determined by a pharmacological approach, both in vivo and in vitro. Results:In vitro, IL-1β production induced by m- and t-CPPD and m-CPPTβ crystals was NLRP3 inflammasome dependent. m-CPPD crystals were the most inflammatory by inducing a faster and higher production and gene expression of IL-1β, IL-6, and IL-8 than t-CPPD, m-CPPTβ and MSU crystals. The a-CPP phase did not show an inflammatory property. Accordingly, m-CPPD crystals led to stronger activation of NF-κB, p38, extracellular signal-regulated kinase 1/2 (ERK1/2) and c-Jun N-terminal kinase (JNK) MAPKs. Inhibition of NF-κB completely abrogated IL-1β and IL-8 synthesis and secretion induced by all CPP crystals. Also, inhibition of JNK and ERK1/2 MAPKs decreased both IL-1β secretion and NF-κB activation induced by CPP crystals. In vivo, IL-1β and CXCL1 production and neutrophil infiltration induced by m-CPPD crystals were greatly decreased by NF-κB inhibitor treatment. Conclusion: Our results suggest that the inflammatory potential of different CPP crystals relies on their ability to activate the MAPK-dependent NF-κB pathway. Studies are ongoing to investigate the underlying mechanisms.
Keywords: CPP crystals; IL-1β; MAP kinase signaling; NF-κB pathway; NLRP3 inflammasome; macrophages; microcrystal-induced arthritis.
Figures




References
-
- Gras P, Rey C, Marsan O, Sarda S, Combes C. Synthesis and characterisation of hydrated calcium pyrophosphate phases of biological interest. Eur J Inorg Chem. (2013) 2013:5886–95. 10.1002/ejic.201300955 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

