iTRAQ-Based Quantitative Proteomic Analysis Reveals Proteomic Changes in Mycelium of Pleurotus ostreatus in Response to Heat Stress and Subsequent Recovery
- PMID: 30356767
- PMCID: PMC6189471
- DOI: 10.3389/fmicb.2018.02368
iTRAQ-Based Quantitative Proteomic Analysis Reveals Proteomic Changes in Mycelium of Pleurotus ostreatus in Response to Heat Stress and Subsequent Recovery
Abstract
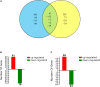
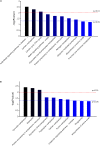
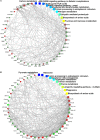
High temperature is a key limiting factor for mycelium growth and development in Pleurotus ostreatus. Thermotolerance includes the direct response to heat stress and the ability to recover from heat stress. To better understand the mechanism of thermotolerance in P. ostreatus, we used morphological and physiological analysis combined with an iTRAQ-based proteomics analysis of P. ostreatus subjected to 40°C for 48 h followed by recovery at 25°C for 3 days. High temperature increased the concentrations of thiobarbituric acid reactive substances (TBARS) indicating that the mycelium of P. ostreatus were damaged by heat stress. However, these physiological changes rapidly returned to control levels during the subsequent recovery phase from heat stress. In comparison to unstressed controls, a total of 204 proteins were changed during heat stress and/or the recovery phase. Wherein, there were 47 proteins that responded to both stress and recovery conditions, whereas 84 and 73 proteins were responsive to only heat stress or recovery conditions, respectively. Furthermore, quantitative real-time PCR (qRT-PCR) confirmed differential expression of nine candidate genes revealed that some of the proteins, such as a mitogen-activated protein kinase (MAPK), phenylalanine ammonia-lyase (PAL), and heat shock protein (HSP), were also regulated by heat stress at the level of transcription. These differentially expressed proteins (DEPs) in mycelium of P. ostreatus under heat stress were from 13 biological processes. Moreover, protein-protein interaction analysis revealed that proteins involved in carbohydrate and energy metabolism, signal transduction, and proteins metabolism could be assigned to three heat stress response networks. On the basis of these findings, we proposed that effective regulatory protein expression related to MAPK-pathway, antioxidant enzymes, HSPs, and other stress response proteins, and glycolysis play important roles in enhancing P. ostreatus adaptation to and recovery from heat stress. Of note, this study provides useful information for understanding the thermotolerance mechanism for basidiomycetes.
Keywords: Pleurotus ostreatus; TBARS; heat stress; proteomics; recovery.
Figures






References
-
- Anandhi R., Annadurai T., Anitha T. S., Muralidharan A. R., Najmunnisha K., Nachiappan V., et al. (2013). Antihypercholesterolemic and antioxidative effects of an extract of the oyster mushroom, Pleurotus ostreatus, and its major constituent, chrysin, in Triton WR-1339-induced hypercholesterolemic rats. J. Physiol. Biochem. 69 313–323. 10.1007/s13105-012-0215-6 - DOI - PubMed
LinkOut - more resources
Full Text Sources

