Cross-Protective Immune Responses Induced by Sequential Influenza Virus Infection and by Sequential Vaccination With Inactivated Influenza Vaccines
- PMID: 30356772
- PMCID: PMC6189474
- DOI: 10.3389/fimmu.2018.02312
Cross-Protective Immune Responses Induced by Sequential Influenza Virus Infection and by Sequential Vaccination With Inactivated Influenza Vaccines
Abstract
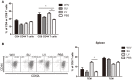
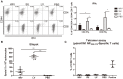
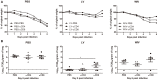
Sequential infection with antigenically distinct influenza viruses induces cross-protective immune responses against heterologous virus strains in animal models. Here we investigated whether sequential immunization with antigenically distinct influenza vaccines can also provide cross-protection. To this end, we compared immune responses and protective potential against challenge with A(H1N1)pdm09 in mice infected sequentially with seasonal A(H1N1) virus followed by A(H3N2) virus or immunized sequentially with whole inactivated virus (WIV) or subunit (SU) vaccine derived from these viruses. Sequential infection provided solid cross-protection against A(H1N1)pdm09 infection while sequential vaccination with WIV, though not capable of preventing weight loss upon infection completely, protected the mice from reaching the humane endpoint. In contrast, sequential SU vaccination did not prevent rapid and extensive weight loss. Protection correlated with levels of cross-reactive but non-neutralizing antibodies of the IgG2a subclass, general increase of memory T cells and induction of influenza-specific CD4+ and CD8+ T cells. Adoptive serum transfer experiments revealed that despite lacking neutralizing activity, serum antibodies induced by sequential infection protected mice from weight loss and vigorous virus growth in the lungs upon A(H1N1)pdm09 virus challenge. Antibodies induced by WIV vaccination alleviated symptoms but could not control virus growth in the lung. Depletion of T cells prior to challenge revealed that CD8+ T cells, but not CD4+ T cells, contributed to cross-protection. These results imply that sequential immunization with WIV but not SU derived from antigenically distinct viruses could alleviate the severity of infection caused by a pandemic and may improve protection to unpredictable seasonal infection.
Keywords: antigenically distinct influenza virus strains; cross-protection; immune mechanism; non-neutralizing antibody; sequential vaccination.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

