Update on Neutrophil Function in Severe Inflammation
- PMID: 30356867
- PMCID: PMC6190891
- DOI: 10.3389/fimmu.2018.02171
Update on Neutrophil Function in Severe Inflammation
Abstract
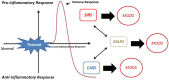
Neutrophils are main players in the effector phase of the host defense against micro-organisms and have a major role in the innate immune response. Neutrophils show phenotypic heterogeneity and functional flexibility, which highlight their importance in regulation of immune function. However, neutrophils can play a dual role and besides their antimicrobial function, deregulation of neutrophils and their hyperactivity can lead to tissue damage in severe inflammation or trauma. Neutrophils also have an important role in the modulation of the immune system in response to severe injury and trauma. In this review we will provide an overview of the current understanding of neutrophil subpopulations and their function during and post-infection and discuss the possible mechanisms of immune modulation by neutrophils in severe inflammation.
Keywords: CD64; infection; innate immunity; neutrophils; severe inflammation; trauma.
Figures



References
-
- Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. (1989) 320:365–76. - PubMed

