PGC-1α and PGC-1β Increase Protein Synthesis via ERRα in C2C12 Myotubes
- PMID: 30356878
- PMCID: PMC6190860
- DOI: 10.3389/fphys.2018.01336
PGC-1α and PGC-1β Increase Protein Synthesis via ERRα in C2C12 Myotubes
Abstract
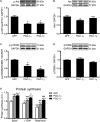
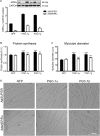
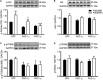
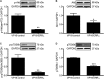
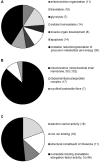
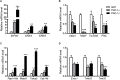
The transcriptional coactivators peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) and PGC-1β are positive regulators of skeletal muscle mass and energy metabolism; however, whether they influence muscle growth and metabolic adaptations via increased protein synthesis is not clear. This study revealed PGC-1α or PGC-1β overexpression in C2C12 myotubes increased protein synthesis and myotube diameter under basal conditions and attenuated the loss in protein synthesis following the treatment with the catabolic agent, dexamethasone. To investigate whether PGC-1α or PGC-1β signal through the Akt/mTOR pathway to increase protein synthesis, treatment with the PI3K and mTOR inhibitors, LY294002 and rapamycin, respectively, was undertaken but found unable to block PGC-1α or PGC-1β's promotion of protein synthesis. Furthermore, PGC-1α and PGC-1β decreased phosphorylation of Akt and the Akt/mTOR substrate, p70S6K. In contrast to Akt/mTOR inhibition, the suppression of ERRα, a major effector of PGC-1α and PGC-1β activity, attenuated the increase in protein synthesis and myotube diameter in the presence of PGC-1α or PGC-1β overexpression. To characterize further the biological processes occurring, gene set enrichment analysis of genes commonly regulated by both PGC-1α and PGC-1β was performed following a microarray screen. Genes were found enriched in metabolic and mitochondrial oxidative processes, in addition to protein translation and muscle development categories. This suggests concurrent responses involving both increased metabolism and myotube protein synthesis. Finally, based on their known function or unbiased identification through statistical selection, two sets of genes were investigated in a human exercise model of stimulated protein synthesis to characterize further the genes influenced by PGC-1α and PGC-1β during physiological adaptive changes in skeletal muscle.
Keywords: C2C12 myotubes; ERRα; PGC-1α; PGC-1β; metabolism; muscle mass; protein synthesis; resistance exercise.
Figures


References
-
- Benton C. R., Nickerson J. G., Lally J., Han X. X., Holloway G. P., Glatz J. F., et al. (2008). Modest PGC-1alpha overexpression in muscle in vivo is sufficient to increase insulin sensitivity and palmitate oxidation in subsarcolemmal, not intermyofibrillar, mitochondria. J. Biol. Chem. 283 4228–4240. 10.1074/jbc.M704332200 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

