Insights into 2D MXenes for Versatile Biomedical Applications: Current Advances and Challenges Ahead
- PMID: 30356929
- PMCID: PMC6193163
- DOI: 10.1002/advs.201800518
Insights into 2D MXenes for Versatile Biomedical Applications: Current Advances and Challenges Ahead
Abstract
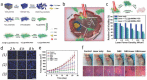
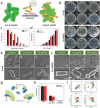
Great and interdisciplinary research efforts have been devoted to the biomedical applications of 2D materials because of their unique planar structure and prominent physiochemical properties. Generally, ceramic-based biomaterials, fabricated by high-temperature solid-phase reactions, are preferred as bone scaffolds in hard tissue engineering because of their controllable biocompatibility and satisfactory mechanical property, but their potential biomedical applications in disease theranostics are paid much less attention, mainly due to their lack of related material functionalities for possibly entering and circulating within the vascular system. The emerging 2D MXenes, a family of ultrathin atomic nanosheet materials derived from MAX phase ceramics, are currently booming as novel inorganic nanosystems for biologic and biomedical applications. The metallic conductivity, hydrophilic nature, and other unique physiochemical performances make it possible for the 2D MXenes to meet the strict requirements of biomedicine. This work introduces the very recent progress and novel paradigms of 2D MXenes for state-of-the-art biomedical applications, focusing on the design/synthesis strategies, therapeutic modalities, diagnostic imaging, biosensing, antimicrobial, and biosafety issues. It is highly expected that the elaborately engineered ultrathin MXenes nanosheets will become one of the most attractive biocompatible inorganic nanoplatforms for multiple and extensive biomedical applications to profit the clinical translation of nanomedicine.
Keywords: MXenes; materials science; nanomedicines; surface chemistry; theranostics.
Figures










References
-
- Ashley C. E., Carnes E. C., Phillips G. K., Padilla D., Durfee P. N., Brown P. A., Hanna T. N., Liu J., Phillips B., Carter M. B., Carroll N. J., Jiang X., Dunphy D. R., Willman C. L., Petsev D. N., Evans D. G., Parikh A. N., Chackerian B., Wharton W., Peabody D. S., Brinker C. J., Nat. Mater. 2011, 10, 389. - PMC - PubMed
-
- Mura S., Nicolas J., Couvreur P., Nat. Mater. 2013, 12, 991. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
