Efficient Water Splitting Cascade Photoanodes with Ligand-Engineered MnO Cocatalysts
- PMID: 30356939
- PMCID: PMC6193156
- DOI: 10.1002/advs.201800727
Efficient Water Splitting Cascade Photoanodes with Ligand-Engineered MnO Cocatalysts
Abstract
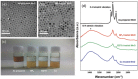
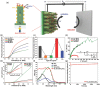
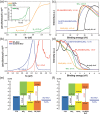
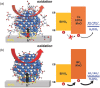
The band edge positions of semiconductors determine functionality in solar water splitting. While ligand exchange is known to enable modification of the band structure, its crucial role in water splitting efficiency is not yet fully understood. Here, ligand-engineered manganese oxide cocatalyst nanoparticles (MnO NPs) on bismuth vanadate (BiVO4) anodes are first demonstrated, and a remarkably enhanced photocurrent density of 6.25 mA cm-2 is achieved. It is close to 85% of the theoretical photocurrent density (≈7.5 mA cm-2) of BiVO4. Improved photoactivity is closely related to the substantial shifts in band edge energies that originate from both the induced dipole at the ligand/MnO interface and the intrinsic dipole of the ligand. Combined spectroscopic analysis and electrochemical study reveal the clear relationship between the surface modification and the band edge positions for water oxidation. The proposed concept has considerable potential to explore new, efficient solar water splitting systems.
Keywords: MnO; band structure; ligand engineering; oxygen evolution catalysts; water splitting.
Figures

References
-
- Dahl S., Chorkendorff I., Nat. Mater. 2012, 11, 100. - PubMed
-
- Gratzel M., Nature 2001, 414, 338. - PubMed
-
- Gray H. B., Nat. Chem. 2009, 1, 7. - PubMed
-
- Lee M. G., Kim D. H., Sohn W., Moon C. W., Park H., Lee S., Jang H. W., Nano Energy 2016, 28, 250.
-
- Lee M. G., Moon C. W., Park H., Sohn W., Kang S. B., Lee S., Choi K. J., Jang H. W., Small 2017, 13, 1701644. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
