C9orf72, a protein associated with amyotrophic lateral sclerosis (ALS) is a guanine nucleotide exchange factor
- PMID: 30356970
- PMCID: PMC6195791
- DOI: 10.7717/peerj.5815
C9orf72, a protein associated with amyotrophic lateral sclerosis (ALS) is a guanine nucleotide exchange factor
Abstract
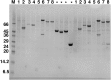
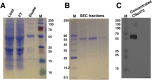
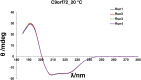
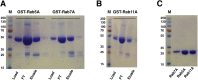
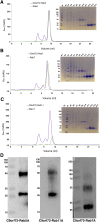
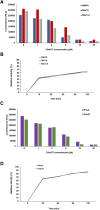
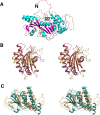
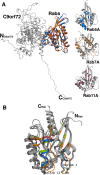
Amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD), two late onset neurodegenerative diseases, have been shown to share overlapping cellular pathologies and genetic origins. Studies suggest that a hexanucleotide repeat expansion in the first intron of the C9orf72 gene is the most common cause of familial FTD and ALS pathology. The C9orf72 protein is predicted to be a differentially expressed in normal and neoplastic cells domain protein implying that C9orf72 functions as a guanine nucleotide exchange factor (GEF) to regulate specific Rab GTPases. Reported studies thus far point to a putative role for C9orf72 in lysosome biogenesis, vesicular trafficking, autophagy and mechanistic target of rapamycin complex1 (mTORC1) signaling. Here we report the expression, purification and biochemical characterization of C9orf72 protein. We conclusively show that C9orf72 is a GEF. The distinctive presence of both Rab- and Rho-GTPase GEF activities suggests that C9orf72 may function as a dual exchange factor coupling physiological functions such as cytoskeleton modulation and autophagy with endocytosis.
Keywords: ALS; C9orf72; Guanine nucleotide exchange factor; Neurodegeneration; Protein expression; Protein purification; Rab GTPases; Size-exclusion chromatography.
Conflict of interest statement
Vasanta Subramanian is an Academic Editor for PeerJ.
Figures


Similar articles
-
C9orf72 ALS-FTD: recent evidence for dysregulation of the autophagy-lysosome pathway at multiple levels.Autophagy. 2021 Nov;17(11):3306-3322. doi: 10.1080/15548627.2021.1872189. Epub 2021 Feb 26. Autophagy. 2021. PMID: 33632058 Free PMC article. Review.
-
C9orf72's Interaction with Rab GTPases-Modulation of Membrane Traffic and Autophagy.Front Cell Neurosci. 2016 Oct 7;10:228. doi: 10.3389/fncel.2016.00228. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27774051 Free PMC article. Review.
-
C9orf72 and smcr8 mutant mice reveal MTORC1 activation due to impaired lysosomal degradation and exocytosis.Autophagy. 2020 Sep;16(9):1635-1650. doi: 10.1080/15548627.2019.1703353. Epub 2019 Dec 26. Autophagy. 2020. PMID: 31847700 Free PMC article.
-
The progress in C9orf72 research: ALS/FTD pathogenesis, functions and structure.Small GTPases. 2022 Jan;13(1):56-76. doi: 10.1080/21541248.2021.1892443. Epub 2021 Mar 5. Small GTPases. 2022. PMID: 33663328 Free PMC article. Review.
-
The product of C9orf72, a gene strongly implicated in neurodegeneration, is structurally related to DENN Rab-GEFs.Bioinformatics. 2013 Feb 15;29(4):499-503. doi: 10.1093/bioinformatics/bts725. Epub 2013 Jan 16. Bioinformatics. 2013. PMID: 23329412 Free PMC article.
Cited by
-
Structure of the C9orf72 ARF GAP complex that is haploinsufficient in ALS and FTD.Nature. 2020 Sep;585(7824):251-255. doi: 10.1038/s41586-020-2633-x. Epub 2020 Aug 26. Nature. 2020. PMID: 32848248 Free PMC article.
-
The Interplay Between Autophagy and RNA Homeostasis: Implications for Amyotrophic Lateral Sclerosis and Frontotemporal Dementia.Front Cell Dev Biol. 2022 Apr 28;10:838402. doi: 10.3389/fcell.2022.838402. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35573690 Free PMC article. Review.
-
C9ORF72 suppresses JAK-STAT mediated inflammation.iScience. 2023 Apr 6;26(5):106579. doi: 10.1016/j.isci.2023.106579. eCollection 2023 May 19. iScience. 2023. PMID: 37250330 Free PMC article.
-
ALS' Perfect Storm: C9orf72-Associated Toxic Dipeptide Repeats as Potential Multipotent Disruptors of Protein Homeostasis.Cells. 2024 Jan 17;13(2):178. doi: 10.3390/cells13020178. Cells. 2024. PMID: 38247869 Free PMC article. Review.
-
Zebrafish as a model organism for neurodegenerative disease.Front Mol Neurosci. 2022 Oct 13;15:940484. doi: 10.3389/fnmol.2022.940484. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36311026 Free PMC article. Review.
References
-
- Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. PHENIX: a comprehensive python-based system for macromolecular structure solution. Acta Crystallographica Section D Biological Crystallography. 2010;66(2):213–221. doi: 10.1107/s0907444909052925. - DOI - PMC - PubMed
-
- Ash PE, Bieniek KF, Gendron TF, Caulfield T, Lin WL, Dejesus-Hernandez M, Van Blitterswijk MM, Jansen-West K, Paul JW, 3rd, Rademakers R, Boylan KB, Dickson DW, Petrucelli L. Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron. 2013;77(4):639–646. doi: 10.1016/j.neuron.2013.02.004. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

