Effect of Human Recombinant Alkaline Phosphatase on 7-Day Creatinine Clearance in Patients With Sepsis-Associated Acute Kidney Injury: A Randomized Clinical Trial
- PMID: 30357272
- PMCID: PMC6248164
- DOI: 10.1001/jama.2018.14283
Effect of Human Recombinant Alkaline Phosphatase on 7-Day Creatinine Clearance in Patients With Sepsis-Associated Acute Kidney Injury: A Randomized Clinical Trial
Abstract
Importance: Sepsis-associated acute kidney injury (AKI) adversely affects long-term kidney outcomes and survival. Administration of the detoxifying enzyme alkaline phosphatase may improve kidney function and survival.
Objective: To determine the optimal therapeutic dose, effect on kidney function, and adverse effects of a human recombinant alkaline phosphatase in patients who are critically ill with sepsis-associated AKI.
Design, setting, and participants: The STOP-AKI trial was an international (53 recruiting sites), randomized, double-blind, placebo-controlled, dose-finding, adaptive phase 2a/2b study in 301 adult patients admitted to the intensive care unit with a diagnosis of sepsis and AKI. Patients were enrolled between December 2014 and May 2017, and follow-up was conducted for 90 days. The final date of follow-up was August 14, 2017.
Interventions: In the intention-to-treat analysis, in part 1 of the trial, patients were randomized to receive recombinant alkaline phosphatase in a dosage of 0.4 mg/kg (n = 31), 0.8 mg/kg (n = 32), or 1.6 mg/kg (n = 29) or placebo (n = 30), once daily for 3 days, to establish the optimal dose. The optimal dose was identified as 1.6 mg/kg based on modeling approaches and adverse events. In part 2, 1.6 mg/kg (n = 82) was compared with placebo (n = 86).
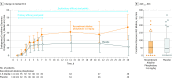
Main outcomes and measures: The primary end point was the time-corrected area under the curve of the endogenous creatinine clearance for days 1 through 7, divided by 7 to provide a mean daily creatinine clearance (AUC1-7 ECC). Incidence of fatal and nonfatal (serious) adverse events ([S]AEs) was also determined.
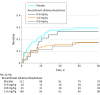
Results: Overall, 301 patients were enrolled (men, 70.7%; median age, 67 years [interquartile range {IQR}, 59-73]). From day 1 to day 7, median ECC increased from 26.0 mL/min (IQR, 8.8 to 59.5) to 65.4 mL/min (IQR, 26.7 to 115.4) in the recombinant alkaline phosphatase 1.6-mg/kg group vs from 35.9 mL/min (IQR, 12.2 to 82.9) to 61.9 mL/min (IQR, 22.7 to 115.2) in the placebo group (absolute difference, 9.5 mL/min [95% CI, -23.9 to 25.5]; P = .47). Fatal adverse events occurred in 26.3% of patients in the 0.4-mg/kg recombinant alkaline phosphatase group; 17.1% in the 0.8-mg/kg group, 17.4% in the 1.6-mg/kg group, and 29.5% in the placebo group. Rates of nonfatal SAEs were 21.0% for the 0.4-mg/kg recombinant alkaline phosphatase group, 14.3% for the 0.8-mg/kg group, 25.7% for the 1.6-mg/kg group, and 20.5% for the placebo group.
Conclusions and relevance: Among patients who were critically ill with sepsis-associated acute kidney injury, human recombinant alkaline phosphatase compared with placebo did not significantly improve short-term kidney function. Further research is necessary to assess other clinical outcomes.
Trial registration: ClinicalTrials.gov Identifier: NCT02182440.
Conflict of interest statement
Figures
Comment in
-
A cure for septic AKI: Why not keep the dream alive?Anaesth Crit Care Pain Med. 2019 Feb;38(1):1-2. doi: 10.1016/j.accpm.2018.12.008. Epub 2019 Jan 8. Anaesth Crit Care Pain Med. 2019. PMID: 30635097 No abstract available.
-
Human recombinant alkaline phosphatase: a promising, yet-to-be-tested agent for the treatment sepsis-induced acute kidney injury.Ann Transl Med. 2018 Dec;6(Suppl 2):S124. doi: 10.21037/atm.2018.12.17. Ann Transl Med. 2018. PMID: 30740445 Free PMC article. No abstract available.
-
Treatment of sepsis-induced acute kidney injury in the ICU: the therapeutic targets do not seem to be established yet.Ann Transl Med. 2019 Sep;7(Suppl 6):S181. doi: 10.21037/atm.2019.07.66. Ann Transl Med. 2019. PMID: 31656760 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

