Prevalence of Drug Prescriptions and Potential Safety in Patients with Cirrhosis: A Retrospective Real-World Study
- PMID: 30357649
- PMCID: PMC6450857
- DOI: 10.1007/s40264-018-0744-1
Prevalence of Drug Prescriptions and Potential Safety in Patients with Cirrhosis: A Retrospective Real-World Study
Abstract
Introduction: Patients with cirrhosis are at risk for adverse drug reactions (ADRs) due to altered pharmacokinetics and pharmacodynamics. We aimed to determine the prevalence of drug prescriptions and the potential safety of these prescriptions in a real-world cohort of patients with cirrhosis.
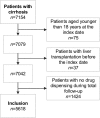
Methods: This was a retrospective cohort study based on linked real-world data from the Out-patient Pharmacy Database and the Hospitalisation Database of the PHARMO Database Network. Patients with a diagnosis of cirrhosis between January 1998 and December 2015 were included. Follow-up ended when the patient underwent a liver transplant, died, transferred out of the database, or on 31 December 2015. Prescription data were derived from a community pharmacy database and were compared with our previously developed safety recommendations for 209 drugs.
Results: In total, 5618 patients were included and followed for a median of 3 years (interquartile range [IQR] 1-7). In the first year after the diagnosis, patients used a median of nine drugs (IQR 5-14), with proton pump inhibitors (prevalence 53.9%), aldosterone antagonists (43.6%), and sulfonamide diuretics (41.3%) being the most commonly used drug groups. Almost half (48.3%) of 102,927 prescriptions consisted of drugs with a safety recommendation. The prevalence of potentially unsafe drug use was 60.0% during the total follow-up. Three nonsteroidal anti-inflammatory drugs (NSAIDs) were among the five most commonly used potentially unsafe drugs.
Conclusions: Patients with cirrhosis use a large number of drugs. Almost two-thirds of patients in our cohort used potentially unsafe drugs. To prevent ADRs in these frail patients, personalised pharmacotherapy is necessary.
Conflict of interest statement
Conflict of interest
Eline Houben is an employee of the PHARMO Institute of Drug Outcomes Research, an independent research institute that performs financially supported studies for government and related healthcare authorities as well as several pharmaceutical companies. Rianne Weersink, Katja Taxis, Joost Drenth, Herold Metselaar and Sander Borgsteede declare that they have no competing interests to declare.
Ethical approval
For this type of study, formal consent is not required.
Availability of data and material
Requests for sharing study data must be made on specific grounds either (1) with the aim of corroborating the study results in the interest of Public Health, or (2) in the context of an audit by a competent authority. Sufficient information needs to be provided to confirm that the request is made for one of the above-mentioned purposes, including a sound justification and, in case of a request with a view to corroborate study results, a protocol on the research for which the data will be used, or a plan for quality-control checks, as applicable.
References
-
- Lucena MI, Andrade RJ, Tognoni G, Hidalgo R, Sanchez de La Cuesta F. Drug use for non-hepatic associated conditions in patients with liver cirrhosis. Eur J Clin Pharmacol. 2003;59:71–6. - PubMed
-
- Lucena MI, Andrade RJ, Tognoni G, Hidalgo R, Sanchez de La Cuesta F. Multicenter hospital study on prescribing patterns for prophylaxis and treatment of complications of cirrhosis. Eur J Clin Pharmacol. 2002;58:435–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases