Should heart rate variability be "corrected" for heart rate? Biological, quantitative, and interpretive considerations
- PMID: 30357862
- PMCID: PMC6378407
- DOI: 10.1111/psyp.13287
Should heart rate variability be "corrected" for heart rate? Biological, quantitative, and interpretive considerations
Abstract
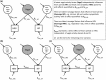
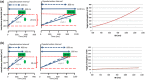
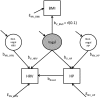
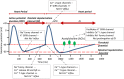
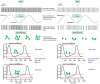
Metrics of heart period variability are widely used in the behavioral and biomedical sciences, although somewhat confusingly labeled as heart rate variability (HRV). Despite their wide use, HRV metrics are usually analyzed and interpreted without reference to prevailing levels of cardiac chronotropic state (i.e., mean heart rate or mean heart period). This isolated treatment of HRV metrics is nontrivial. All HRV metrics routinely used in the literature exhibit a known and positive relationship with the mean duration of the interval between two beats (heart period): as the heart period increases, so does its variability. This raises the question of whether HRV metrics should be "corrected" for the mean heart period (or its inverse, the heart rate). Here, we outline biological, quantitative, and interpretive issues engendered by this question. We provide arguments that HRV is neither uniformly nor simply a surrogate for heart period. We also identify knowledge gaps that remain to be satisfactorily addressed with respect to assumptions underlying existing HRV correction approaches. In doing so, we aim to stimulate further progress toward the rigorous use and disciplined interpretation of HRV. We close with provisional guidance on HRV reporting that acknowledges the complex interplay between the mean and variability of the heart period.
Keywords: autonomic; behavioral medicine; heart rate; heart rate variability.
© 2018 The Authors Psychophysiology published by Wiley Periodicals, Inc. on behalf of Society for Psychophysiological Research.
Figures

References
-
- Abildstrom, S. Z. , Jensen, B. T. , Agner, E. , Torp‐Pedersen, C. , Nyvad, O. , Wachtell, K. , … Kanters, J. K. (2003). Heart rate versus heart rate variability in risk prediction after myocardial infarction. Journal of Cardiovascular Electrophysiology, 14(2), 168–173. 10.1046/j.1540-8167.2003.02367.x - DOI - PubMed
-
- Allen, J. J. B. , & Chambers, A. S. (2007). Special issue of biological psychology on cardiac vagal control, emotion, psychopathology, and health. Biological Psychology, 74(2), 113–115. - PubMed
-
- Anrep, G. V. , Pascual, F. R. S. W. , & Roessler, R. (1936). Respiratory variations of the heart rate. Proceedings of the Royal Society B Biological Sciences, 119(813), 191–217. 10.1098/rspb.1936.0005 - DOI
Publication types
MeSH terms
Grants and funding
- UL1TR001881/TR/NCATS NIH HHS/United States
- 1UL1RR025011/NH/NIH HHS/United States
- UL1 TR001409/TR/NCATS NIH HHS/United States
- HL089850/NH/NIH HHS/United States
- U19 AG051426/AG/NIA NIH HHS/United States
- F32 HL137227/HL/NHLBI NIH HHS/United States
- UL1TR001409/TR/NCATS NIH HHS/United States
- P01-AG020166/AG/NIA NIH HHS/United States
- U19-AG051426/AG/NIA NIH HHS/United States
- T32 HL082610/HL/NHLBI NIH HHS/United States
- UL1 RR025011/RR/NCRR NIH HHS/United States
- F32 HL137227/NH/NIH HHS/United States
- R01 HL101959/HL/NHLBI NIH HHS/United States
- HL101959/NH/NIH HHS/United States
- UL1 TR002373/TR/NCATS NIH HHS/United States
- P01 AG020166/AG/NIA NIH HHS/United States
- UL1 TR001881/TR/NCATS NIH HHS/United States
- R01 HL089850/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources

