Photoinducible Oncometabolite Detection
- PMID: 30358041
- PMCID: PMC8141106
- DOI: 10.1002/cbic.201800651
Photoinducible Oncometabolite Detection
Abstract
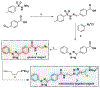
Dysregulated metabolism can fuel cancer by altering the production of bioenergetic building blocks and directly stimulating oncogenic gene-expression programs. However, relatively few optical methods for the direct study of metabolites in cells exist. To address this need and facilitate new approaches to cancer treatment and diagnosis, herein we report an optimized chemical approach to detect the oncometabolite fumarate. Our strategy employs diaryl tetrazoles as cell-permeable photoinducible precursors to nitrileimines. Uncaging these species in cells and cell extracts enables them to undergo 1,3-dipolar cycloadditions with endogenous dipolarophile metabolites such as fumarate to form pyrazoline cycloadducts that can be readily detected by their intrinsic fluorescence. The ability to photolytically uncage diaryl tetrazoles provides greatly improved sensitivity relative to previous methods, and enables the facile detection of dysregulated fumarate metabolism through biochemical activity assays, intracellular imaging, and flow cytometry. Our studies showcase an intersection of bioorthogonal chemistry and metabolite reactivity that can be applied for biological profiling, imaging, and diagnostics.
Keywords: bioorthogonal chemistry; cycloaddition; epigenetics; fluorescent detection; metabolism.
© 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

