An Investigation into Rigidity-Activity Relationships in BisQAC Amphiphilic Antiseptics
- PMID: 30358105
- PMCID: PMC6440202
- DOI: 10.1002/cmdc.201800622
An Investigation into Rigidity-Activity Relationships in BisQAC Amphiphilic Antiseptics
Abstract
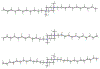
Twenty-one mono- and biscationic quaternary ammonium amphiphiles (monoQACs and bisQACs) were rapidly prepared in order to investigate the effects of rigidity of a diamine core structure on antiseptic activity. As anticipated, the bioactivity against a panel of six bacteria including methicillin-resistant Staphylococcus aureus (MRSA) strains was strong for bisQAC structures, and is clearly correlated with the length of non-polar side chains. Modest advantages were noted for amide-containing side chains, as compared with straight-chained alkyl substituents. Surprisingly, antiseptics with more rigidly disposed side chains, such as those in DABCO-12,12, showed the highest level of antimicrobial activity, with single-digit MIC values or better against the entire bacterial panel, including sub-micromolar activity against an MRSA strain.
Keywords: antiseptics; benzalkonium chloride; bisQAC; methicillin-resistant Staphylococcus aureus (MRSA); quaternary ammonium compounds.
© 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
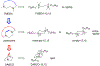
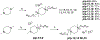
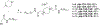

References
-
- Block SS, Disinfection, Sterilization, and Preservation, Lea & Febiger, Philadelphia, 1991, pp. 167–181.
-
- Gibbs FW, Ann. Sci 1939, 4, 169–190.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

