Somatic Gene Editing of GUCY2D by AAV-CRISPR/Cas9 Alters Retinal Structure and Function in Mouse and Macaque
- PMID: 30358434
- PMCID: PMC6534089
- DOI: 10.1089/hum.2018.193
Somatic Gene Editing of GUCY2D by AAV-CRISPR/Cas9 Alters Retinal Structure and Function in Mouse and Macaque
Abstract
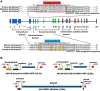
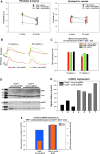
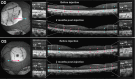
Mutations in GUCY2D, the gene encoding retinal guanylate cyclase-1 (retGC1), are the leading cause of autosomal dominant cone-rod dystrophy (CORD6). Significant progress toward clinical application of gene replacement therapy for Leber congenital amaurosis (LCA) due to recessive mutations in GUCY2D (LCA1) has been made, but a different approach is needed to treat CORD6 where gain of function mutations cause dysfunction and dystrophy. The CRISPR/Cas9 gene editing system efficiently disrupts genes at desired loci, enabling complete gene knockout or homology directed repair. Here, adeno-associated virus (AAV)-delivered CRISPR/Cas9 was used specifically to edit/disrupt this gene's early coding sequence in mouse and macaque photoreceptors in vivo, thereby knocking out retGC1 expression and demonstrably altering retinal function and structure. Neither preexisting nor induced Cas9-specific T-cell responses resulted in ocular inflammation in macaques, nor did it limit GUCY2D editing. The results show, for the first time, the ability to perform somatic gene editing in primates using AAV-CRISPR/Cas9 and demonstrate the viability this approach for treating inherited retinal diseases in general and CORD6 in particular.
Keywords: AAV; CRISPR/Cas9; GUCY2D; cone rod dystrophy; gene editing; retina.
Conflict of interest statement
S.E.B. and P.D.G. received funding from Editas Medicine to support these studies. Their role in this project was to provide gRNAs, perform indel analysis on treated retinas, and evaluate T-cell responses in PBMCs isolated from macaques. S.G., S.H., S.S., H.J., and M.L.M. are employees and shareholders of Editas Medicine.
Figures



References
-
- Gregory-Evans K, Kelsell RE, Gregory-Evans CY, et al. Autosomal dominant cone–rod retinal dystrophy (CORD6) from heterozygous mutation of GUCY2D, which encodes retinal guanylate cyclase. Ophthalmology 2000;107:55–61 - PubMed
-
- Dizhoor AM, Lowe DG, Olshevskaya EV, et al. The human photoreceptor membrane guanylyl cyclase, RetGC, is present in outer segments and is regulated by calcium and a soluble activator. Neuron 1994;12:1345–1352 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

