Acute pulmonary effects of aerosolized nicotine
- PMID: 30358437
- PMCID: PMC6383503
- DOI: 10.1152/ajplung.00564.2017
Acute pulmonary effects of aerosolized nicotine
Abstract
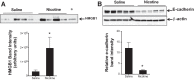
Nicotine is a highly addictive principal component of both tobacco and electronic cigarette that is readily absorbed in blood. Nicotine-containing electronic cigarettes are promoted as a safe alternative to cigarette smoking. However, the isolated effects of inhaled nicotine are largely unknown. Here we report a novel rat model of aerosolized nicotine with a particle size (~1 μm) in the respirable diameter range. Acute nicotine inhalation caused increased pulmonary edema and lung injury as measured by enhanced bronchoalveolar lavage fluid protein, IgM, lung wet-to-dry weight ratio, and high-mobility group box 1 (HMGB1) protein and decreased lung E-cadherin protein. Immunohistochemical analysis revealed congested blood vessels and increased neutrophil infiltration. Lung myeloperoxidase mRNA and protein increased in the nicotine-exposed rats. Complete blood counts also showed an increase in neutrophils, white blood cells, eosinophils, and basophils. Arterial blood gas measurements showed an increase in lactate. Lungs of nicotine-inhaling animals revealed increased mRNA levels of IL-1A and CXCL1. There was also an increase in IL-1α protein. In in vitro air-liquid interface cultures of airway epithelial cells, there was a dose dependent increase in HMGB1 release with nicotine treatment. Air-liquid cultures exposed to nicotine also resulted in a dose-dependent loss of barrier as measured by transepithelial electrical resistance and a decrease in E-cadherin expression. Nicotine also caused a dose-dependent increase in epithelial cell death and an increase in caspase-3/7 activities. These results show that the nicotine content of electronic cigarettes may have adverse pulmonary and systemic effects.
Keywords: aerosol; lung injury; nicotine; pulmonary edema; rats.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures







References
-
- Ahmad A, Ahmad S, Glover L, Miller SM, Shannon JM, Guo X, Franklin WA, Bridges JP, Schaack JB, Colgan SP, White CW. Adenosine A2A receptor is a unique angiogenic target of HIF-2alpha in pulmonary endothelial cells. Proc Natl Acad Sci USA 106: 10684–10689, 2009. doi: 10.1073/pnas.0901326106. - DOI - PMC - PubMed
-
- Ahmad S, Ahmad A, Dremina ES, Sharov VS, Guo X, Jones TN, Loader JE, Tatreau JR, Perraud AL, Schöneich C, Randell SH, White CW. Bcl-2 suppresses sarcoplasmic/endoplasmic reticulum Ca2+-ATPase expression in cystic fibrosis airways: role in oxidant-mediated cell death. Am J Respir Crit Care Med 179: 816–826, 2009. doi: 10.1164/rccm.200807-1104OC. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

