Phenotypic Screens Reveal Posaconazole as a Rapidly Acting Amebicidal Combination Partner for Treatment of Primary Amoebic Meningoencephalitis
- PMID: 30358879
- PMCID: PMC6420171
- DOI: 10.1093/infdis/jiy622
Phenotypic Screens Reveal Posaconazole as a Rapidly Acting Amebicidal Combination Partner for Treatment of Primary Amoebic Meningoencephalitis
Abstract
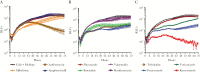
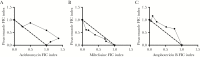
Naegleria fowleri is the causative agent of primary amoebic meningoencephalitis (PAM), which is fatal in >97% of cases. In this study, we aimed to identify new, rapidly acting drugs to increase survival rates. We conducted phenotypic screens of libraries of Food and Drug Administration-approved compounds and the Medicines for Malaria Venture Pathogen Box and validated 14 hits (defined as a 50% inhibitory concentration of <1 μM). The hits were then prioritized by assessing the rate of action and efficacy in combination with current drugs used to treat PAM. Posaconazole was found to inhibit amoeba growth within the first 12 hours of exposure, which was faster than any currently used drug. In addition, posaconazole cured 33% of N. fowleri-infected mice at a dose of 20 mg/kg and, in combination with azithromycin, increased survival by an additional 20%. Fluconazole, which is currently used for PAM therapy, was ineffective in vitro and vivo. Our results suggest posaconazole could replace fluconazole in the treatment of PAM.
Keywords: Naegleria fowleri; azithromycin; drug discovery; miltefosine; phenotypic screen; posaconazole; primary amoebic meningoencephalitis.
© The Author(s) 2018. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures



References
-
- Yoder JS, Eddy BA, Visvesvara GS, Capewell L, Beach MJ. The epidemiology of primary amoebic meningoencephalitis in the USA, 1962–2008. Epidemiol Infect 2010; 138:968–75. - PubMed
-
- Visvesvara GS, Stehr-Green JK. Epidemiology of free-living ameba infections. J Protozool 1990; 37:25S–33S. - PubMed
-
- Capewell LG, Harris AM, Yoder JS, et al. . Diagnosis, clinical course, and treatment of primary amoebic meningoencephalitis in the United States, 1937–2013. J Pediatric Infect Dis Soc 2015; 4:68–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

