Phase Ib/II Study of Pembrolizumab and Pegylated-Interferon Alfa-2b in Advanced Melanoma
- PMID: 30359157
- PMCID: PMC6286160
- DOI: 10.1200/JCO.18.00632
Phase Ib/II Study of Pembrolizumab and Pegylated-Interferon Alfa-2b in Advanced Melanoma
Abstract
Purpose: Objective responses are reported in 34% to 37% of patients with programmed death-1 (PD-1)-naïve advanced melanoma treated with PD-1 inhibitors. Pre-existing CD8+ T-cell infiltrate and interferon (IFN) gene signature correlate with response to PD-1 blockade. Here, we report a phase Ib/II study of pembrolizumab/pegylated (PEG)-IFN combination in PD-1-naïve advanced melanoma.
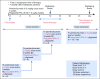
Patients and methods: PEG-IFN (1, 2, and 3 μg/kg per week) was dose escalated using a modified toxicity probability interval design in three cohorts of four patients each, whereas pembrolizumab was dosed at 2 mg/kg every 3 weeks in the phase Ib portion. Thirty-one patients were enrolled in the phase II portion. Primary objectives were safety and incidence of dose-limiting toxicities. Secondary objectives included objective response rate, progression-free survival (PFS), and overall survival.
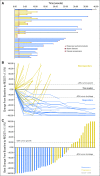
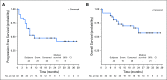
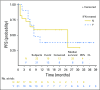
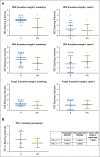
Results: Forty-three patients with stage IV melanoma were enrolled in the phase Ib and II portions of the study and included in the analysis. At the data cutoff date (December 31, 2017), median follow-up duration was 25 months (range, 1 to 38 months). All 43 patients experienced at least one adverse event; grade 3/4 treatment-related adverse events occurred in 21 of 43 patients (48.8%). Objective responses were seen at all three dose levels among 43 evaluable patients. The objective response rate was 60.5%, with 46.5% of patients exhibiting ongoing response. Median PFS was 11.0 months in all patients and unreached in responders, whereas median overall survival remained unreached in all patients. The 2-year PFS rate was 46%.
Conclusion: Pembrolizumab/PEG-IFN demonstrated an acceptable toxicity profile with promising evidence of clinical efficacy in PD-1-naïve metastatic melanoma. These results support the rationale to further investigate this pembrolizumab/PEG-IFN combination in this disease.
Figures



References
-
- Larkin J, Lao CD, Urba WJ, et al. : Efficacy and safety of nivolumab in patients with BRAF V600 mutant and BRAF wild-type advanced melanoma: A pooled analysis of 4 clinical trials. JAMA Oncol 1:433-440, 2015 - PubMed
-
- Ribas A, Hamid O, Daud A, et al. : Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA 315:1600-1609, 2016 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

