Patient-reported outcomes in urticarial vasculitis treated with omalizumab: case report
- PMID: 30359231
- PMCID: PMC6203196
- DOI: 10.1186/s12895-018-0077-x
Patient-reported outcomes in urticarial vasculitis treated with omalizumab: case report
Abstract
Background: Despite the current knowledge of UV, there is a lack of consensus among diagnostic criteria and management. In general, antihistamine therapy is regularly used for the symptomatic management of pruritus but does not control inflammation or alter the course of the disease. Monoclonal antibodies such as omalizumab (anti-IgE) have been proposed as a potential treatment for urticarial vasculitis. A few studies have reported the benefits of omalizumab in patient-reported outcome measures (PROMs). Herein we describe a female patient with urticarial vasculitis who was treated with omalizumab. We discuss the response to treatment and possible implications of PROMs in guiding the management of the disease.
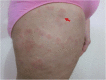
Case presentation: We describe the case of a 57-year-old woman with a diagnosis of urticarial vasculitis. Due to lack of response to first-line treatment and the severity of the disease, treatment with omalizumab was initiated. Omalizumab 150 mg was administered every four weeks for three months. Second-generation antihistamines were used as needed. Both CU-Q2oL and UAS 7 improved. After three-month therapy with omalizumab, disease severity improved from moderate severity (UAS7 = 19) to well controlled (UAS7 = 6). However, 5 months after the last administration of omalizumab, the patient complained of worsening symptoms and active disease with quality of life impairment. A single dose of omalizumab (150 mg) was prescribed with corticosteroids. Thereafter, the patient presented a disease activity and quality of life with a fluctuating pattern that was controlled with additional doses of omalizumab.
Conclusion: In chronic urticaria, patient-reported outcome measures (PROMs) are important for assessing disease status and the impact of symptoms on patients' lives. However, to our knowledge, there is no validated tool to measure such outcomes in UV patients. Although UAS7 and CU-Q2oL were not designed for UV assessment, they might be useful in the clinical setting as objective measures to determine treatment efficacy. However, some domains in the CU-Q2oL questionnaires do not correlate well with UAS7, which might serve as a relative indication to continue treatment despite disease severity improvement. Based on our observations, we believe omalizumab 150 mg might be a feasible therapeutic alternative when first-line treatment is unsuccessful.
Keywords: Omalizumab; Patient-reported outcomes; Urticarial vasculitis.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable
Consent for publication
Written informed consent was obtained from the patient for publication of this Case report and all accompanying images. A copy of the written consent is available for review by the Series Editor of this journal if requested.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



Similar articles
-
Improvement in the quality of life of patients with chronic spontaneous urticaria treated with omalizumab in real life.Enferm Clin. 2017 Nov-Dec;27(6):361-368. doi: 10.1016/j.enfcli.2017.03.010. Enferm Clin. 2017. PMID: 28457893 English, Spanish.
-
Effectiveness of omalizumab in chronic spontaneous urticaria assessed with patient-reported outcomes: a prospective study.J Eur Acad Dermatol Venereol. 2018 Oct;32(10):1761-1767. doi: 10.1111/jdv.15045. Epub 2018 May 29. J Eur Acad Dermatol Venereol. 2018. PMID: 29729103 Clinical Trial.
-
Successful treatment of urticarial vasculitis with omalizumab in children: a case series.Clin Exp Dermatol. 2023 Sep 19;48(10):1145-1148. doi: 10.1093/ced/llad192. Clin Exp Dermatol. 2023. PMID: 37227921
-
Successful treatment of normocomplementemic urticarial vasculitis with omalizumab: A report of three cases and literature review.Asian Pac J Allergy Immunol. 2020 Dec;38(4):286-289. doi: 10.12932/AP-050918-0402. Asian Pac J Allergy Immunol. 2020. PMID: 30660172 Review.
-
Chronic Urticaria: An Overview of Treatment and Recent Patents.Recent Pat Inflamm Allergy Drug Discov. 2019;13(1):27-37. doi: 10.2174/1872213X13666190328164931. Recent Pat Inflamm Allergy Drug Discov. 2019. PMID: 30924425 Free PMC article. Review.
Cited by
-
Real-World Experience on Omalizumab Treatment for Patients with Normocomplementemic Urticarial Vasculitis.J Asthma Allergy. 2021 Apr 23;14:433-437. doi: 10.2147/JAA.S304099. eCollection 2021. J Asthma Allergy. 2021. PMID: 33935505 Free PMC article.
-
Urticarial vasculitis.Int J Womens Dermatol. 2021 Jan 29;7(3):290-297. doi: 10.1016/j.ijwd.2021.01.021. eCollection 2021 Jun. Int J Womens Dermatol. 2021. PMID: 34222586 Free PMC article. Review.
-
Challenging Clinical Therapeutic Approach to Urticarial Vasculitis: A Case Series.J Clin Med. 2025 Jun 27;14(13):4580. doi: 10.3390/jcm14134580. J Clin Med. 2025. PMID: 40648952 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

