Update: Influenza Activity - United States and Worldwide, May 20-October 13, 2018
- PMID: 30359347
- PMCID: PMC6290813
- DOI: 10.15585/mmwr.mm6742a3
Update: Influenza Activity - United States and Worldwide, May 20-October 13, 2018
Abstract
During May 20-October 13, 2018,* low levels of influenza activity were reported in the United States, with a mix of influenza A and B viruses circulating. Seasonal influenza activity in the Southern Hemisphere was low overall, with influenza A(H1N1)pdm09 predominating in many regions. Antigenic testing of available influenza A and B viruses indicated that no significant antigenic drift in circulating viruses had emerged. In late September, the components for the 2019 Southern Hemisphere influenza vaccine were selected and included an incremental update to the A(H3N2) vaccine virus used in egg-based vaccine manufacturing; no change was recommended for the A(H3N2) component of cell-manufactured or recombinant influenza vaccines. Annual influenza vaccination is the best method for preventing influenza illness and its complications, and all persons aged ≥6 months who do not have contraindications should receive influenza vaccine, preferably before the onset of influenza circulation in their community, which often begins in October and peaks during December-February. Health care providers should offer vaccination by the end of October and should continue to recommend and administer influenza vaccine to previously unvaccinated patients throughout the 2018-19 influenza season (1). In addition, during May 20-October 13, a small number of nonhuman influenza "variant" virus infections† were reported in the United States; most were associated with exposure to swine. Although limited human-to-human transmission might have occurred in one instance, no ongoing community transmission was identified. Vulnerable populations, especially young children and other persons at high risk for serious influenza complications, should avoid swine barns at agricultural fairs, or close contact with swine.§.
Conflict of interest statement
All authors have completed and submitted the ICMJE form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
Figures
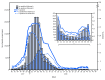
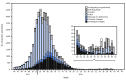

References
-
- Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2018–19 influenza season. MMWR Recomm Rep 2018;67(No. RR-3). 10.15585/mmwr.rr6703a1 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

