Xylose metabolism in the pig
- PMID: 30359396
- PMCID: PMC6201911
- DOI: 10.1371/journal.pone.0205913
Xylose metabolism in the pig
Abstract
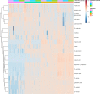
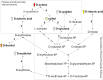
It is important to understand if, and to what extent, the pig can utilize xylose as an energy source if xylanase releases free xylose in the small intestine. The experimental objectives were to determine the effects of industry-relevant dietary xylose concentrations and adaptation time on xylose retention efficiency and metabolism, diet digestibility and energy value, nitrogen balance, and hindgut fermentation. Forty-eight pigs were housed in metabolism crates and randomly assigned to one of four treatments with increasing D-xylose levels (n = 12/treatment) in 2 replications of a 22-d experiment with 3 collection periods. The control diet was xylose-free (0%), to which either 2, 4, or 8% D-xylose was added. Adaptation effects were assessed during three fecal and urine collection periods: d 5-7, 12-14, and 19-21. On d 22, pigs from the 0 and 8% treatments were euthanized; cecal and colon digesta were collected. Dietary xylose did not affect the total tract digestibility of dry matter, gross energy, or crude protein (P>0.10). Digesta short chain fatty acids concentrations and molar proportions and cecal pH were not different (P>0.10). This experiment utilized a targeted metabolomics approach to characterize and quantify urine xylose and metabolite excretion. Xylose retention decreased from 60% to 47% to 41% when pigs were fed diets containing 2, 4, or 8% xylose, respectively. In the 4 and 8% treatments, xylose retention was greater in the 2nd and 3rd collection periods compared to the 1st. A comprehensive pathway for xylose metabolism was proposed and D-threitol was confirmed as the major urinary metabolite of xylose. In conclusion, pigs can metabolize xylose, but with considerably lower efficiency than glucose, and may be able to adapt with time to utilize xylose more efficiently.
Conflict of interest statement
The commercial funding received in support of this study, namely Elanco Animal Health and IPPA does not alter our adherence to PLOS ONE policies on sharing data and materials. We suggest there is no need to list the in-kind financial support as it represents very little value towards the actual cost of the experiment. However, in keeping with PLOS ONE policy, the in-kind contributions of Danisco, DSM Nutritional Products and Ajinomoto North America did not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

References
-
- Paloheimo M, Piironen J, Vehmaanperä J. Xylanases and cellulases as feed additives In: Bedford MR, Partridge GG, editors. Enzymes in Farm Animal Nutrition. 2nd ed. Oxfordshire, UK: CABI; 2010. pp. 12–53.
-
- Biely P, Vrsanka M, Kucar S. Identification and mode of action of endo-(1–4)-β-xylanases In: Visser J, Beldman G, Kusters-van Someren MA, Voragen AGJ, editors. Xylans and Xylanases (Progress in Biotechnology). 7th ed. Amsterdam, The Netherlands: Elsevier; 1992. pp. 81–94.
-
- Coughlan MP. Towards an understanding of the mechanism of action of main chain-hydrolyzing xylanases In: Visser J, Beldman G, Kusters-van Someren MA, Voragen AGJ, editors. Xylans and Xylanases (Progress in Biotechnology). 7th ed. Amsterdam, The Netherlands: Elsevier; 1992. pp. 111–140.
-
- NRC. Nutrient requirements of swine. 11th revis. Washington, DC: National Academy Press; 2012.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

