Aortic stiffness, pressure and flow pulsatility, and target organ damage
- PMID: 30359540
- PMCID: PMC6842890
- DOI: 10.1152/japplphysiol.00108.2018
Aortic stiffness, pressure and flow pulsatility, and target organ damage
Abstract
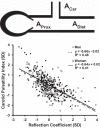
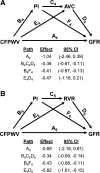
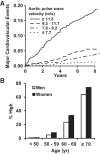
Measures of aortic stiffness and pressure and flow pulsatility have emerged as correlates of and potential contributors to cardiovascular disease, dementia, and kidney disease. Higher aortic stiffness and greater pressure and flow pulsatility are associated with excessive pulsatile load on the heart, which increases mass and reduces global longitudinal strain of the left ventricle. Excessive stiffness and pulsatility are also associated with microvascular lesions in high-flow organs, such as the brain and kidney, suggesting that small vessels in these organs are damaged by pulsatility. This brief review will summarize evidence relating aortic stiffness to cardiovascular, brain, and kidney disease.
Keywords: aorta; cardiovascular disease; chronic kidney disease; dementia; hemodynamics; pulsatility index.
Conflict of interest statement
G. F. Mitchell is owner of Cardiovascular Engineering, Inc., a company that designs and manufactures devices that measure vascular stiffness. He has received recent grants or consulting fees from Novartis, Servier, and Merck.
Figures

References
-
- Anderson TJ, Charbonneau F, Title LM, Buithieu J, Rose MS, Conradson H, Hildebrand K, Fung M, Verma S, Lonn EM. Microvascular function predicts cardiovascular events in primary prevention: long-term results from the Firefighters and Their Endothelium (FATE) Study. Circulation 123: 163–169, 2011. doi: 10.1161/CIRCULATIONAHA.110.953653. - DOI - PubMed
-
- Andersson C, Quiroz R, Enserro D, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Mitchell GF, Vasan RS. Association of parental hypertension with arterial stiffness in nonhypertensive offspring: the Framingham Heart Study. Hypertension 68: 584–589, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07426. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

